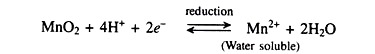
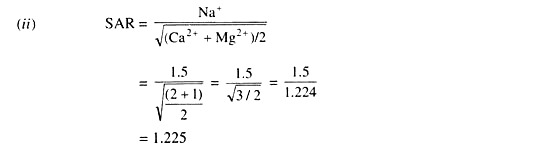ADVERTISEMENTS:
This article throws light upon the top three nitrogenous fertilizers applied to soil. The nitrogenous fertilizers are: 1. Urea 2. Ammonium Sulphate 3. Calcium Ammonium Nitrate.
Nitrogenous Fertilizer # 1. Urea:
Manufacture:
Ammonia reacts with carbon dioxide under a pressure of about 400 atmospheres, at a temperature of about 160 to 200°C to form urea.
Properties:
Urea is a white granular organic compound which contains 46.67 per cent N. Fertilizer urea contains 46 per cent N. It is soluble in water.
(i) On heating, urea loses a molecule of ammonia to form biuret which is poisonous substance.
For detecting biuret in urea an aqueous solution of urea is treated with sodium hydroxide solution and a drop of very dilute copper sulphate solution is added.
ADVERTISEMENTS:
A violet colouration is produced if biuret is present in it.
(ii) Urea reacts with nitrous acid to produce nitrogen gas NH,
(iii) When urea is boiled with dil. acid, alkali or water under pressure or when it is treated with the enzyme urease it decomposes to produce ammonia.
Urea slowly decomposes to ammonia as shown in the above equation at a temperature of 80 to 90°F and relative humidity of 50-75 per cent. This decomposition of urea is accelerated in the presence of mono-calcium phosphate. Hence urea should not be mixed with single super phosphate.
Reaction in Soil:
When urea is added to the soil it is decomposed by the enzymes urease produced by micro-organisms to ammonium carbonate which is oxidized to nitric acid as shown below:
This nitric acid then reacts with free calcium carbonate molecules if they are present in the soil and also with the clay and the humic micelle as shown in the following equations:
This calcium nitrate is leached by high rainfall in the humid region when the above reaction proceeds in the forward direction. Therefore if urea is continuously applied to the soil for a long time, the clay and humic micelle is gradually saturated with hydrogen ion and ultimately the soil becomes acidic in reaction if there is insufficient calcium carbonate.
This soil acidity can be neutralized by adding 80kgs of limestone for each 100 kg of urea. If rainfall is not high then calcium nitrate accumulates in the soil and is assimilated by crops. It may be applied at the sowing time or as top dressing to most soils, but should not be allowed to come in contact with the seed.
Nitrogenous Fertilizer # 2. Ammonium Sulphate:
ADVERTISEMENTS:
(i) Manufacture from coal:
Coal contains about one per cent nitrogen. When it is heated in a closed retort in the absence of air ammonia and ammonia salts are formed. Slaked lime is then added to it. When it is reheated ammonia is evolved, which is absorbed in 60 per cent sulphuric acid, obtained by product from industries.
(ii) Synthetic Process:
When Nitrogen and Hydrogen in the ratio of 1: 3 are passed over heated (500°C) platinized asbestos or powdered iron at a pressure of 250 atmospheres, ammonia is formed which is passed through a suspension of gypsum in water through which carbon dioxide is also passes.
Calcium carbonate is removed by filtration. An aqueous solution of ammonium sulphate is evaporated when crystals of ammonium sulphate is formed which is cried and bagged.
Properties:
Fertiliser ammonium sulphate is white to yellowish grey in colour. It contains about 20.5 per cent Nitrogen and 23.5 per cent Sulphur. It is neutral to litmus.
It reacts with mono-calcium phosphate of single super phosphate, potassium chloride (Muriate of Potash) and calcium carbonate in the presence of moisture as shown in the following equation.
Ammonium sulphate, on heating, decomposes as shown in the equation below:
Reaction in Soils:
When ammonium sulphate is applied to the soil, ammonium ions replace basic cations from the clay and humic micelle as shown in the following equation.
These absorbed ammonium ions are oxidized to nitrate as shown below:
The nitric acid then reacts with the calcium carbonate and the clay and humic micelle as shown below:
In the humid region, calcium nitrate is leached by high rainfall; the above reaction proceeds in the forward direction when the clay and humic micelle is gradually saturated with hydrogen ions; ultimately the soil becomes acidic in reaction, if it does not contain calcium carbonate.
Soil acidity is caused by adding 100kgs of ammonium sulphate can be neutralized by adding 110 kg of limestone. Some ammonium ions are fixed by certain clay minerals like illite, vermiculate etc.
Nitrogenous Fertilizer # 3. Calcium Ammonium Nitrate (Nitro-Chalk or A.N.L.)
Manufacture:
When a mixture of Nitrogen and Hydrogen in the ratio of 1: 3 is passed over heated (500° C) platinized asbestos at a pressure of about 250 atmospheres, ammonia is formed. N2 + 3H2—– > 2NH3. A part of this ammonia is mixed with air and passed over a red hot platinum gauge and nitric acid is formed.
The nitric acid then reacts with the reaming ammonia to form nitrate.
NH3 + HNO3 Ã NH4NO3
The ammonium nitrate solution still contains some nitric acid which is neutralized by adding limestone
Properties:
Calcium ammonium nitrate is a brown or light grey. It contains 20.5 per cent Nitrogen, one-fourth of which is ammoniacal, and three-fourths, nitrate. Calcium ammonium nitrate containing 25 per cent nitrogen is now being manufactured.
Reaction in soil:
It reacts with soil as the ammonium sulphate does, but the soil does not become acidic in reaction even if there is no reserve calcium carbonate as shown in the following equation.
This nitric acid reacts with calcium carbonate present in the soil:
If the soil does not contain free calcium carbonate this nitric acid reacts with the clay and humic micelle.
Since the soil contains enough calcium nitrate, the above reaction does not proceed in the forward direction. Hence the soil does not become acidic in reaction. Calcium nitrate is assimilated by crops.