ADVERTISEMENTS:
This article throws light upon the three main types of chemical fertilizers available in the market. The types are: 1. Nitrogenous Fertilizers 2. Phosphatic Fertilizers 3. Potassic Fertilizers.
Chemical Fertilizers: Type # 1. Nitrogenous Fertilizers:
Nitrogenous fertilizers are those fertilizers that are sold for their nitrogen content.
Classification:
ADVERTISEMENTS:
Nitrogenous fertilizers can be classified into four classes on the basis of forms of N present in straight nitrogenous fertilizers as follows:
1. Nitrate nitrogen (NO3-N) containing fertilizers e.g. NaNO3-16% N; Ca(NO3)2— 15.5% N.
2. Ammonium containing nitrogenous fertilizers (NH4-N), e.g. (NH4)2SO4-20% N; NH4Cl—24 to 26% N, anhydrous ammonia, 82% N;
3. Both NH4 and NO3-N containing nitrogenous fertilizers; e.g. ammonium nitrate (NH4NO3)—33 to 34% N Calcium ammonium nitrate (CAN)—20% N.
ADVERTISEMENTS:
4. Amide fertilizers—It is organic form of N-containing fertilizers, e.g. Urea [CO(NH2)2]—46% N, Calcium Cyanamide (CaCN2)—21% N.
The most important and widely used nitrogenous fertilizers are ammonium sulphate and urea.
(i) Ammonium sulphate [(NH4)2SO4]:
Ammonium sulphate about 20% N. It is a white crystalline salt, stable and soluble in water. It can he stored well. The solution form of (NH4)2SO4 is acidic to litmus. In this fertilizer, Nitrogen is present in cationic form (NH). So this form of N can be retained by soil colloids. Consequently loss of N through leaching is less.
ADVERTISEMENTS:
It is less hygroscopic and therefore, this possesses fewer problems in handling. All N-fertilizers containing N as NH4 form or fertilizers which after application in soil produce NH4 ion, are physiologically acidic in character. It is not subject to loss by denitrification. The application of heavy dose of (NH4)2SO4 or any other NH4 fertilizers in alkaline soils may lead to the loss of N through volatilization.
Reactions in Soils:
(i) In upland soil conditions. When (NH4)2SO4 is applied to the upland soil, the first reaction is the cation-exchange, in which ammonium (NH4+) displaces some other cations, usually calcium, on the exchange complex of the soil.
The reaction can be represented as follows:
Calcium sulphate [CaSO4], so formed, may be leached and lost in drainage water, particularly in a humid climate. In this way the loss of soil calcium (Ca2+) takes place due to application of (NH4)2SO4 fertilizer. The ammonium (NH4+) on the soil colloid may be taken up directly by the plants or it is nitrified ![]() . The biological process by which oxidation of such NH4+ to NO3– is known as nitrification. It is a two-step process in which the ammonium (NH4+) is first converted to nitrite (NO2–) and the two nitrates (NO3–).
. The biological process by which oxidation of such NH4+ to NO3– is known as nitrification. It is a two-step process in which the ammonium (NH4+) is first converted to nitrite (NO2–) and the two nitrates (NO3–).
The above reaction indicated that hydrogen (H+) ions are formed during nitrification which results in the acidification of the soil.
The sulphate portion of (NH4)2SO4 combines with Ca2+ to form CaSO4. Calcium sulphate being soluble in water is partly absorbed by plants, but mainly it is leached through the soil during heavy rains. Thus loss of CaSO4 occurs in soil.
ADVERTISEMENTS:
Continued use of (NH4)2SO4 will lower the soil pH. The speed and extent of such transformation will be profoundly influenced by soil environment like moisture regimes and temperatures. s a result of continued application of (NH4)2SO4 depletion of exchangeable bases as well as release of H+ in the soil solution occurs and that also leads to the development of soil acidity.
That is why NH4+—fertilizers are called physiologically acidic fertilizers. If a soil contains large amounts of free CaCO3, then the loss of Ca2+ will fall upon the CaCO3 and not on the exchangeable bases. Under such conditions, there will be no development of acidity due to (NH4)2SO4 application.
In Upland Calcareous Soils:
When ammonium sulphate is applied to calcareous soils considerable losses of ammonia through volatilization may occur.
The ammonium carbonate. (NH4)2CO3, is unstable and decomposes easily to give NH3, CO2 and H2O. The losses of nitrogen from (NH4)2SO4, applied as broadcast in calcareous soils, may be appreciable. But when (NH4)2SO4 is drilled, the losses of nitrogen are not likely to be appreciable.
Volatilization of Ammonia:
Ammonium containing [(NH4)2SO4] or forming (urea) fertilizers will react with CaCO3 in soil to form (NH4)2CO3 and calcium precipitates.
The following general equations may be represented for the above mentioned reactions:
X(NH4)2 Y + N Ca CO3→ N(NH4)2CO3 + CanYx … (1)
where Y = anion combined with NH ions
N, X and Z are dependent on the valences of the anion and cation.
The final reaction product. (NH4)2CO3 is unstable and decomposes as follows:
The amount of NH4OH formed in a given time would depend on the solubility of CanYx and its rate of formation. If CanYx is insoluble, the reaction will proceed to the right resulting more (NH4)2 CO3 and consequently more NH4OH is to be formed. If no insoluble precipitate is formed, no appreciable amount of (NH4) CO3 will exist.
When (NH4)2CO3 decomposes according to second equation (2) CO2 is lost from solution at a faster rate than NH3 thereby producing additional OH– ions and an increase in the concentration of OH– ions. Consequently, more solution NH4+ becomes electrically balanced by OH” ions which will favour NH3 loss as follows:
NH4+ + OH–DNH4OH DNH3↑ + H2O
This type of reaction occurs when nitrogenous fertilizers like (NH4)2SO4, urea etc. are applied in calcareous and alkali soils.
(ii) Urea [CO(NH2)2]:
Urea is an organic solid nitrogenous fertilizer. It contains about 46 per cent N. Urea is manufactured by reacting anhydrous NH3 and CO2 gas under very high pressure in the presence of a suitable catalyst.
The reaction is as follows:
This unstable intermediate product is decomposed and urea is recovered. The urea solution is then concentrated to 99 per cent and is sprayed into a chamber where urea crystals are formed.
Urea is a white crystalline substance. At 86° F urea absorbs moisture from the atmosphere if the relative humidity is 72 per cent or above and releases moisture to the atmosphere if the humidity is below 72 per cent.
During concentrating the solution or crystallization or for preparation of granulated urea at high temperature, some amount of biuret may be formed by condensation reaction as follows:
This biuret is toxic to the plant. Biuret concentration in urea should not exceed 1.5 per cent. When urea will be applied as foliar spray on crop canopy, the biuret concentration should not exceed 0.25 per cent. Urea is less acidic compared to (NH4)2SO4. Pure solution of urea is slowly hydrolysed into NH3 and CO2 in absence of bacteria or enzyme.
But this reaction is accelerated or enhanced at high temperature in combination with the addition of alkali or acid and also by adding bacteria and enzyme urease. Urea reacts with HN02 releasing elemental N and C02. Acid equivalent of urea is 84.
Reactions of Urea in Soil:
After application of urea in soil, it undergoes enzymatic hydrolysis to produce an unstable compound designated as ammonium carbamate.
This NH3 is converted to NH4+ ions by accepting one proton (H+) from proton donor and subsequently forms NH4OH or any other NH compound depending upon the nature of the donor. After formation of NH4—compound, its behaviour in soil and effect on the soil property will be similar to that of any other N-fertilizers as ammoniacal form.
In neutral, slightly alkaline and calcareous soils, the chances of formation of nitrites (NO2–) are more than in acid soils. When urea is applied in relatively high amounts, nitrites accumulate even in acid soils, because the pH of the soil increases during the hydrolysis of urea.
In high concentration of urea, there is a combined toxic effect of NH3 and NO2 (nitrite). Due to higher concentration of NH3, the conversion of NH4→ NO2 will increase. But due to lower population of nitrobacter, the conversion of NO2→ NO3 will be inhibited.
Advantages of Urea over Ammonium sulphate:
(i) During the manufacturing process of urea, no raw materials are to be imported; therefore, no foreign exchange is involved. Whereas for ammonium sulphate, (NH4)2SO4, gypsum or sulphur may be imported which involves foreign exchange.
(ii) Urea is a solid organic—N fertilizer containing highest percentage of N. So, for urea cost of transport and handling per unit weight of N is less than (NH4)2SO4.
(iii) Urea is relatively less.-acid producing fertilizer that (NH4)2SO4.
(iv) Urea can be used carefully for foliar application, but ammonium sulphate cannot be used as foliar application.
Slow Release Nitrogenous Fertilizers:
The efficiency of synthetic inorganic N—fertilizers hardly exceeds 60-70 per cent. The rest amount is lost from the root zone by different mechanisms. The maximum amount is lost by the process of leaching in the form of nitrate. Therefore, attempts have been made to produce N-fertilizer materials which release N slowly in available form or to develop materials which control the release of N in available form slowly.
The manufacturing of this slow release N- fertilizers have the following advantages:
(i) Slow release N-fertilizers minimise the loss of N increases the efficiency of the fertilizers.
(ii) It helps to avoid frequent application of N-fertilizers.
(iii)It helps to prevent different harmful effects of plants like germination of seeds and emergence of seedlings.
(iv) It avoids the luxury consumption of N and prevents the imbalances due to different nutrients within the plants.
Classification:
The slow release-N-fertilizers can be mainly classified as follows:
(a) Conventional soluble N-fertilizers coated with some other materials.
(b) N-substances of low water solubility.
(c) Nitrification and urease inhibitors.
(d) Sparingly soluble minerals.
(a) Coated N-Fertilizers:
The most important coated N-fertilizers that the used as slow releasing N fertilizers are sulphur coated urea (SCU), neem coated urea (NCU), lac coated urea (LCU) etc. Due to coating with neem cakes, lac or sulphur, urea comes into the soil solution through diffusion process very slowly and in this way they supply nitrogen to the plants at a controlled rate or slow rate but for a longer period.
In addition, neem cake and lac contains alkaloids which inhibits the activities of nitrifying bacteria. As a result, the rate of nitrification is minimised and the leaching loss of N is reduced. In case of sulphur coated urea, the elemental sulphur which is placed in the soil along with the fertilizers is oxidised by S-oxidising organisms to sulphuric acid (H2SO4).
As a result, acidity increases in the micro-zone of the fertilizer resulting reduced activity of the nitrifying bacteria. Thus, the rate of nitrification is controlled and N loss as nitrate (NO3) is minimised.
(b) N-Substances of Low Water Solubility:
A large number of chemicals have been developed which can be used as slow release N-fertilizers that are given below:
(i) Urea-Formaldehyde or Urea-Form:
Urea-formaldehyde compounds are the major group of compounds that are commercially available. They are white, odorless solids analyzing about 38 per cent N which are made by reacting with formaldehyde in the presence of a catalyst.
A typical urea form may contain 30 per cent of its nitrogen in forms that are soluble in cold water (25°C). Nitrogen in the cold-water fraction nitrifies almost quickly as urea. Solubility in hot boiling water is a measure of the quality of the remaining 70 per cent of its nitrogen.
An activity index is used by the Association of Official Agricultural Chemists to evaluate the suitability of urea-form compounds.
AI = %CWIN – %HWIN/%CWIN × 100
where, AI = Activity index
CWIN = % N insoluble in cold water at 25°C.
HWIN = % N insoluble in hot water (98 to 100°C)
Therefore, the quality of urea-form may be judged as follows:
(i) The quantity of cold water insoluble N which is the amount of N that will be released slowly,
(ii) The quality of the cold water insoluble N which will indicate the rate at which the insoluble N will release into available form.
(ii) Crotonylidene Diurea (CDU):
This slow-acting nitrogen compound is formed by the reaction of urea’s with croton aldehyde or acetaldehyde. Powdered CDU containing 30 per cent N has been directly used as a fertilizer.
The microbial transformation of chemically bound nitrogen in CDU is known to be temperature dependent.
Besides these, there are various other such N-containing slow acting fertilizers like, thiourea, (NH2)2CS, 36.8% N; urea-Z (reaction product of urea and acetaldehyde, 33-38 per cent N); Oxamide![]() non-hygroscopic, 31.8 per cent N, etc. Oxamide is di-amide of oxalic acid and slowly releases ammonium (NH4) by hydrolysis.
non-hygroscopic, 31.8 per cent N, etc. Oxamide is di-amide of oxalic acid and slowly releases ammonium (NH4) by hydrolysis.
(iii) Nitrification Inhibitors:
A nitrification inhibitor should ideally:
(i) Be non-toxic to plants, soil, micro-organisms, animals, fish and mammals.
(ii) Block the conversion of NH4+→ NO3– by inhibiting Nitrosomonas activity.
(iii) Not interfere with the transformation of NO2– (nitrite) by Nitrobacter,
(iv) Be able to move with the fertilizer or fertilizer solution so that it will be distributed uniformly throughout the soil zone contacted by nitrogen fertilizer,
(v) Be stable for its inhibitory action to last for an adequate period of time,
(vi) Be relatively inexpensive, so that it can be used on a commercial basis.
There are various nitrification inhibitors, of which N-serve or nitrapyrin and AM are most important.
N-Serve:
It is 2-chloro-6 (trichloromethyl) pyridine and also referred to as nitrapyrin.
AM:
Chemically it is a substituted pyrimidine (2-amino-4-chloro-6-methyl- pyrimidine).
Chemical Fertilizers: Type # 2. Phosphatic Fertilizers:
The plant nutrient content of all phosphatic fertilizers is expressed in terms of percentage of phosphorus rather than the percentage of P2O5.
The conversion of % P to % P2O5 and vice- versa (% P2O5 to % P) is simple and the following expressions are used:
% P = % P2Os× 0.43
% P2O5 = % P × 2.29
This conversion should be used because most of the commercial grade phosphatic fertilizers are marketed as % P2O5 content. Phosphorus has generally three forms namely P2O5 (Phosphorus pentaoxide in burning form of P), HPO3 (Metaphosphoric acid, when dissolves in water) and H3PO4 (Orthophosphoric acid when dissolves in warm water). But the salts of H3PO4 are most important and it has three replaceable H+ ions.
Plants absorb phosphorus is H2PO4– and HPO42- forms. Three replaceable ions of H3PO4 combine with Ca2+ to form three different combined salts of calcium and phosphorus resulting different classes of phosphatic fertilizers.
Classification:
Phosphatic fertilizers can be classified into three groups on the basis of forms in which orthophosphoric acid (H3PO4) is combined with calcium (Ca2+);
(i) Water soluble mono-calcium phosphate, [Ca(H2PO4)2]
Example:
Superphosphates (SSP, DSP and TSP)
Single Superphosphate (SSP)—16-18% P2O5 or 6.88-7.74% P
Double Superphosphate (DSP) —32% P2O5 or 13.76% P
Triple Superphosphate (TSP) — 46-48% P2O5 or 19.78-20.64% P
They contain water soluble phosphorus and can be easily available to plants as H2P04 ions. However, within a very short time this class of fertilizers is converted into insoluble phosphorus when it is applied to the soil. The magnitude of such reaction with the soil depends on the nature and properties of soil.
(ii) Citric acid soluble, di-calcium phosphate. [Ca2H2(PO4)2, or CaHPO4]
Example:
Basic slag, silicates of lime,—14-18% P2O5 or 6.02-7.74% P
Dicalcium phosphate—34-39% P2O5 or 14.62-16.77%
This class of fertilizers is not readily soluble in water and hence not readily available to plants. But this class of fertilizers is suitable for acidic soils.
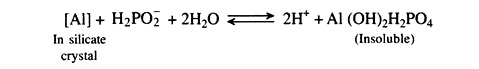
(iii) Phosphatic fertilizers not soluble in water or not soluble in citric acid, tricalcium phosphate, [Ca3(PO4)2].
Example:
Rock phosphate, 20-40% P2O5 or 8.6—17.2% P
Raw bone meal, 20-25% P2O5 or 8.6-10.75% P
Steamed bone meal, 22% P2O5 or 9.46% P
This class of fertilizers is suitable for strongly acidic soils or organic soils (peat). In India, most important phosphatic fertilizers used by the farmers are superphosphates and rock phosphates.
Reactions in Soils:
Effectiveness of phosphatic fertilizers is determined by the properties of both the phosphorus salt and the soil being fertilized and by the reactions which occur between the phosphorus fertilizer and various soil constituents.
Superphosphate:
Superphosphate is formed due to the reaction of rock phosphates and conc. H2SO4 as follows:
When superphosphate is applied to a moist or to a dry soil before rainfall or irrigation, the mono calcium phosphate gets dissolved in the soil moisture. The roots of the growing plants easily take up this form of phosphorus. But within a very short time, the solution of mono calcium phosphate is precipitated in the soil pores.
Depending on the soil reaction, different soil-fertilizer reaction products are formed that are not soluble in water and becomes unavailable to plants. Thus superphosphate is not leached from the soil by the rainfall.
When superphosphate is applied to strong acid soils, it combines with iron and aluminium to form their respective insoluble phosphates and also di-calcium phosphate as follows:
In this way, phosphate becomes unavailable to plants.
When superphosphate is applied to moderately acid soils, phosphate fixation takes place by the silicate minerals present in soil and becomes unavailable to plants. Aluminium and iron are removed from the edges of the silicate crystals forming hydroxy phosphates.
A part of the reacted phosphate with Fe and AI compounds is also released by anion exchange reactions with OH– ions.
Again, when superphosphate is applied to neutral and calcareous soils, the following reaction will take place and P becomes unavailable to plants.
The insoluble tri-calcium phosphate thus formed may be converted in the soil to more insoluble compounds like hydroxyl apatite, Ca10(PO4)5(OH)4; carbonatoapatite, Ca10(PO4)6(CO3)2; and fluorapatite, Ca10(PO4)6. F2 and ultimately ‘P’ becomes unavailable to plants.
When superphosphate is applied to alkaline soils (pH > 8.5 or 9.0, Na+ is the dominant cation), it reacts with Na+ and forms mono-sodium phosphate which is highly soluble and becomes available to plants.
H2PO4 + Na+DNaH2PO4 (Soluble)
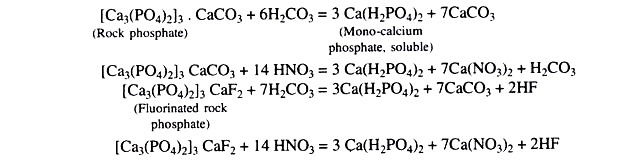
Rock Phosphate:
It is stable and quite insoluble in water. The citrate solubility varies from 5 to about 17 per cent of the total phosphorus content. Effectiveness of rock-phosphate (tri-calcium phosphate) is determined by its chemical reactivity which in turn depends on the degree of carbonate substitution for phosphate in the apatite structure.
Finely ground apatite phosphate rocks are effective only on acid soil (pH 6 or below). But calcined iron-aluminium phosphate ores with much higher citrate solubilities 60-65 per cent of the ‘P’ can be used successfully on neutral and calcareous soils.
In situations where the reactivity of available rock phosphate is inadequate partially acidulated rock phosphate (treating rock phosphate with H2SO4) can be used successfully.
A granular material containing mixture of raw rock phosphate and finely ground elemental sulphur has been developed and designated as “Bio super”. It is inoculated with the sulphur-oxidising bacteria thiohacillusthioxidans to ensure the conversion of sulphur to sulphuric acid (S → H2SO4).
This acid in turn reacts with the rock phosphate and releases soluble phosphorus in the soil and thereby increases ‘P’ availability to plants. When rock phosphate is applied to acidic soils and high amount of organic matter, the following reactions may take place due to formation of H2CO3 and HNO3arising from the decomposition of organic matter.
Chemical Fertilizers: Type # 3. Potassic Fertilizers:
Recently the supply of potassium to plants is expressed as K+ (potassium) percentage instead of K2O percentage in potassic fertilizers.
Converting percentage K to percentage K2O and vice-versa can be accomplished as follows:
% K = % K2O× 0.83
% K2O = % K × 1.2
Practically all of the potassium fertilizers are water soluble. Different potassic fertilizers essentially consist of potassium in combination with chloride, sulphate, nitrate or polyphosphates etc.
Muriate of Potash (KCI):
The term muriate is derived from muriatic acid, a common name for hydrochloric acid. Fertilizer grade muriate contains 50-52 per cent potassium (60-63 per cent K2O) and varies in colour from pink or red to white depending on the mining and recovery process used. Muriate of potash is generally marketed in five particles sizes: special standard, white soluble, standard, coarse and granular, of which coarse and granular size fractions of muriate of potash are widely used.
Muriate of potash is manufactured from potash-bearing minerals namely carnallite, KCl.MgCl2.6H2O (17.0 per cent K2O); Sylvite, KCI, Sylvinite, KCI, NaCl mixture, etc. Recovery of potassium chloride (KCI) from sylvinite ore is made by the mineral floatation process or by solution of KCI, followed by recrystallization. Floatation process is based on the differences in the specific gravities of KCI and NaCl.
The potassium chloride having lesser specific gravity floatation the top of NaCl and thereby KCI is separated out. Whereas in the re-crystallization process, the difference in temperature-solubility relationships of chloride salts of K and Na is the fundamental principle of this method. The solubility of KCI increases rapidly with a rise in temperature, whereas NaCl solubility varies only slightly over a wide temperature range.
Sulphate of Potash (K2SO4):
Potassium sulphate is a white salt which contains 41.5 to 44.2 per cent K (50-53.2 per cent K2O). It is produced by number of processes like, Langbeinite, Trona, Mannheim, Glaserite processes etc.
Langbeinite Process:
Production form Langbeinite minerals (K2SO4.2MgSO4, 22.6% K2O), occurs according to the following reactions:
K2SO4.2MgSO4 + 4KCl→ 3K2SO4 + 2MgCl2
Trona Process:
Burkite (Na2CO3.2Na2SO4) is reacted with potassium chloride to form glaserite (Na2SO4.3K2SO4) and then reacted with KCI brine to give potassium sulphate.
Galserite Process:
The following reactions take place in the production of sulphate of potash from sodium sulphate (Na2SO4) and KCI with glaserite as an intermediate product.
Reactions in Soils:
Both chloride and sulphate of K are soluble in water and on application to the soil they ionize into K+, Cl– and SO42- ions. The released K+ ion from the fertilizer gets adsorbed on the soil colloids and also available to the plant through cation exchange reactions.
Based on the chemistry of chloride in the soils, it is concluded that under acidic soil conditions CI– ion replaces the OH– ions associated with the free iron oxides and therefore, in such soils, muriate of potash is likely to give a greater response than K2SO4. Besides, the Cl– ions are less strongly retained on soil colloids than the SO42- ions.
In alkaline soils, when muriate of potash (KCI) is applied, the accumulation of CI– ions creates toxic to plants. So in potash deficient soils with alkaline reaction, it should be applied along with organic matter. The application of potassic fertilizers has little or no effect on soil reaction.