ADVERTISEMENTS:
This article throws light upon the four important types of silicate minerals. The types are: 1. Orthosilicates 2. Inosilicates 3. Phyllosilicates 4. Tectosilicates.
Silicate Minerals: Type # 1. Orthosilicates:
Represented by olivine—(Fe, Mg)2 SiO4: The structure consists of individual silicon-tetrahedra (SiO4)4- alternating with positively charged metal ions (Fe, Mg) which balance the negative charge of the tetrahedra units.
The close packing in olivines is achieved by having the tetrahedra alternately pointing to opposite directions, for without inter tetrahedral bonding there is no stability to preserve an open structure. The close packing is reflected in the high density of olivine compared with other silicates. The mineral is named after its typical olive colour and is without cleavage.
Silicate Minerals: Type # 2. Inosilicates:
ADVERTISEMENTS:
Represented by pyroxenes and amphiboles: The silicon-tetrahedra join together forming long chains. These are of two kinds:
—single-chain inosilicates, represented by pyroxenes (SiO3)2-.
—double-chain inosilicates, represented by amphiboles (Si4O11)6-.
The crystalline structure of pyroxene group is based on single chain of tetrahedra which are bonded to one another by positive metallic ions of iron, magnesium and calcium. The mineral develops cleavage along two planes almost at right angles.
ADVERTISEMENTS:
The crystalline structure of amphiboles, (double-chain inosilicates) is based on double chains of tetrahedra (see Fig. 2.4) joined together by iron and magnesium ions common to all ferromagnesian and also by ions of calcium, sodium and aluminium as there is a certain amount of replacement of silicon by aluminium. The common minerals in this group are hornblende, tremolite and actinolite.
Silicate Minerals: Type # 3. Phyllosilicates:
The phyllosilicates are an important group of soil-forming minerals and are represented by the micas (biotite, muscovite). They have sheet structure of tetrahedra (see Fig. 2.4) where each silicon ion shares three oxygen ions with adjacent silicon ion to form a pattern like honey-comb. The fourth unshared oxygen ion of each tetrahedron stands above the plane of all others.
The basic structural unit of phyllosilicates is essentially formed by the condensation of two sheets of silicon-tetrahedra with one sheet of aluminium or magnesium octahedron. Aluminium atoms normally occupy only two-thirds of the available octahedral positions in the aluminium octahedron sheet giving rise to the characteristic mineral structure of gibbsite [Al2(OH)6]n.
If instead of aluminium, magnesium is present, then all the available octahedral positions are filled, giving rise to brucite [Mg3(OH)6]n structure. The complete plate-like 2: 1 lattice constitutes regular staking of crystalline layer in the vertical direction which makes up a crystal.
ADVERTISEMENTS:
The crystalline layers of micas (muscovite and biotite) are loosely joined together by positively-charged ions of potassium along which cleavage readily takes place.
In biotite, the positively-charged ions holding inner surfaces are iron and magnesium. In muscovite, each pair of tetrahedra sheets is tightly cemented together by ions of aluminium rather than iron and magnesium.
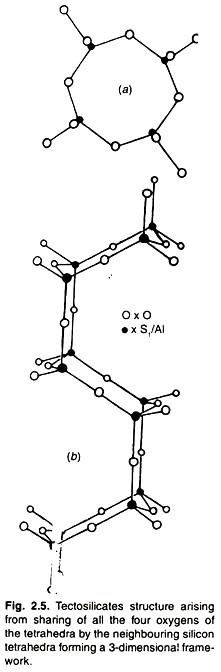
Silicate Minerals: Type # 4. Tectosilicates:
In its structure, all the four oxygen’s of the silica tetrahedron are shared by the neighbouring silicon-tetrahedra. This means that there are two ions of oxygen for every ion of silicon thus forming a 3-dimensional framework.
It is represented by the formula SiO2. In contrast to the other types of Si—O—Si linkage (already discussed) in which the framework exists in only one form, the tectosilicate framework can take various forms. The most common minerals of this group are quartz and feldspars.
ADVERTISEMENTS:
i. Quartz:
The framework of quartz is very densely packed and occurs in a high degree of purity. It is strongly resistant to physical and chemical weathering as the structure is densely packed, electrically neutral and prevents any form of substitution.
With time, it accumulates in soils as the other susceptible minerals decompose to form clay and decrease in their respective amounts. As such, the abundance of quartz in most soils is next only to feldspars.
ii. Feldspars:
ADVERTISEMENTS:
In contrast, the framework in majority of tectosilicates is far more-open and makes them less dense than quartz and other silicates (ortho-, ino- and phyllo-). Feldspars are most abundant among rock-forming minerals and constitute nearly 61 per cent of the minerals that are found in the earth’s crust.
They are non-ferromagnesian minerals and act as storehouse of sodium, calcium, potassium and many trace elements in soils.
In feldspars, the basic structure (Fig. 2.5) is of ring type and made up of four tetrahedra. In two of the tetrahedra, the apical oxygen’s point upwards while in the other two, the oxygen points downwards. Hence each tetrahedron shares its oxygen ions with adjoining tetrahedra ions in a 3-dimensional network.
However, in one-quarter to one-half of the tetrahedra, aluminium ions with a radius of 0.51 Ã… and an electric charge of 3+ have replaced charge of 4+) in the centre of the tetrahedra. The resulting negative charge from such substitution in their structures is balanced by ions, like K+, Na+ or Ca++.
Depending upon the presence of diagnostic ions, like K, Na and Ca, which balance the electric charge, feldspars, are named as orthoclase, albite, anorthite, respectively.
In orthoclase (KAlSi3O8), Al3+ replaces Si4+ in every fourth tetrahedron and K+ corrects the electric imbalance. In albite, (Na Al Si3O8), aluminum replaces silicon in every fourth tetrahedron and Na+ corrects the electric imbalance. In anorthite (CaAl2 Si2O8), however, aluminum replaces silicon in every second tetrahedron and Ca2+ corrects the resulting electric negative charge.