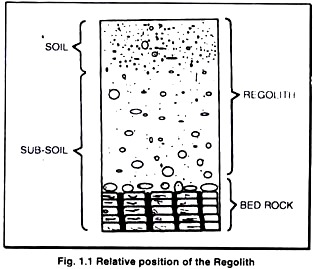
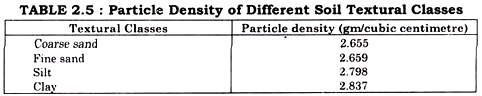
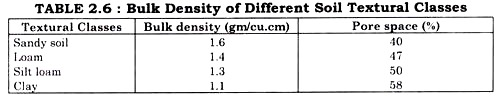
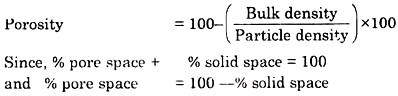ADVERTISEMENTS:
After reading this article you will learn about:- 1. Definition and Broad Classification of Minerals 2. General Constitution of Minerals 3. Important Soil Forming Primary Minerals 4. Fundamental Concepts of Identification 5. Description and Composition of Rock/Soil Forming Minerals.
Definition and Broad Classification of Minerals:
A mineral is a naturally occurring inorganic material that has fairly definite internal structure, composition and properties. Soil forming minerals have been broadly grouped as primary and secondary minerals. Primary minerals originate in the parent rock.
Some of them remain unchanged during the course of soil formation. They are usually present in the sand fraction. Secondary minerals have formed from the alteration and decomposition of primary minerals and subsequent recombination of the products of their decomposition. They are also called clay minerals because they are the chief constituent of clay.
General Constitution of Minerals:
ADVERTISEMENTS:
Ions systematically occur to make the basic building blocks of crystals of minerals under natural conditions. Most of the minerals are made up of silica tetrahedral (singular-tetrahedron) and aluminum octahedral (singular- octahedron). A few of them are built up of silica tetrahedral only.
One silicon ion occupies the central space between four oxygen ions that are closely packed to form a silica tetrahedron. A tetrahedron is a geometrical figure that has four surfaces as shown diagrammatically in Fig. 2.1 B.
All the basal oxygen’s of one silica tetrahedron have been shared by silicon ions of the neighboring silica tetrahedral to make a continuous silica tetrahedral layer of minerals. One aluminum or magnesium ion occupies the central space between six hydroxyl ions that one closely packed to form an aluminum or magnesium octahedron which has eight surfaces. The aluminum octahedron is called a gibbsite.
ADVERTISEMENTS:
The magnesium octahedron is called a brucite. Gibbsite has been diagrammatically represented in Fig. 2.1A. All the hydroxyl ions of one aluminum/magnesium octahedron have been shared by the aluminum/ magnesium ions of the neighboring octahedral to make a continuous sheet of aluminum/magnesium octahedral layer of minerals.
These silica tetrahedral and aluminum/magnesium octahedral are joined together to form minerals, when the top oxygen of the silica tetrahedron occupies the position of one of the hydroxyl ions of the aluminum/magnesium octahedron.
One aluminum octahedron has been shown to be joined with one silica tetrahedron from below in Fig. 2.1C and from below and top in Fig. 2.1D. Some silicon ions are replaced from the tetrahedral position of some minerals by aluminum ions.
Some aluminum ions are replaced from the octahedral position by magnesium, ferrous, ferric and zinc ions. Minerals become negatively changed as a consequence of the above process and therefore attract positively charged cations.
Important Soil Forming Primary Minerals:
ADVERTISEMENTS:
A few very important soil forming primary minerals which are the constituent of important soil forming rocks are briefly described below:
Feldspars:
Feldspars consist of a continuous three-dimensional framework of silica tetrahedra where all the four oxygen’s of one silica tetrahedron have been shared by the silicon ions of the neighboring silica tetrahedra. In feldspar, some of the silica ions of some silica tetrahedra have been replaced by silicon and aluminum ions (Fig. 2.2).
As valencies of silicon and aluminum are four and three respectively, one positively charge is less for each such replacement. So potassium, calcium and sodium ions enter the structure to balance it electrically. Therefore feldspars have been divided into the following groups.
Potassium, sodium and calcium feldspars are called orthoclase, Albite and Anorthite respectively while labradorite is sodium-calcium feldspar.
Pyroxenes:
Pyroxenes consist of single chains of silica tetrahedra in which every two basal oxygen ions of each silica tetrahedron are shared by silicon ions of the neighboring silica tetrahedra as shown in Fig. 2.3A.
The unsatisfied negative charges of the third basal oxygen ions and the top oxygen ions are satisfied by mostly by calcium, magnesium and ferrous ions which connect one pyroxene chain with another.
ADVERTISEMENTS:
A dark coloured mineral called Augite of formula Ca2 (AlFe)4 (Mg Fe)4 Si6 O24, belongs to the pyroxene group of minerals which also include hypersthene and enstatite.
Amphiboles:
Amphiboles consist of a double chain of silica tetrahedra where every two and every three basal oxygen ions of each silica tetrahedron are alternately shared by silicon ions of the neighboring silica tetrahedra as shown in Fig. 2.3B.
The unsatisfied negative charge of the third basal oxygen ions and the top oxygen ions are satisfied mostly by calcium, magnesium and ferrous ions which connect one amphibole chain with the another. A dark coloured mineral, hornblende of formula Ca2Al2Mg2Fe3Si6O22 (OH)2 belongs to the amphibole group
Olivine:
Olivine consists of independent silica tetrahedra in which oxygen ions of one silica tetrahedron are not shared by the silicon ions of the neighboring silica tetrahedra. The unsatisfied negative charge of the oxygen ions of one silica tetraheaion are satisfied by magnesium and ferrous ions which connect one silica tetrahedron with another.
Mica:
In the case of mica, one aluminum octahedral layer has been joined with two silica tetrahedral layers, one at the top and the other at the bottom as shown in Fig. 2.4A and B when the top oxygen ions of the silica tetrahedral layers replaces one of the hydroxyl ions of the aluminum octahedral layers.
So these oxygen ions are common to tetrahedral and octahedral layer as shown in Fig. 2.4A and B. One-fourth of the silicon ions have been replaced from the tetrahedral position by aluminum ions. The valency of Si4+ is 4 and valency of Al3+ is 3.
Hence there is one positive charge less for replacement of each silicon ion with one aluminum ion. Thus potassium ion occurs between the sheets of mica to balance it electrically.
When two-thirds of the octahedral positions are occupied by aluminum ions and one-third remains empty as shown in Fig. 2.4B, the mica is called white mica, muscovite or di-octahedral mica. When all the octahedral positions are occupied by magnesium and/or ferrous ions, it is called black mica, biotite or trioctahedral mica as shown in Fig. 2.4A.
Quartz:
It consists of a continuous, three-dimensional framework of silica tetrahedra when all the four oxygen ions of each silica tetrahedron have been shared by the silicon ions of the neighboring silica tetrahedra as shown in Fig. 2.5A. All the negative charges of the oxygen ions are satisfied by silicon ions themselves. Hence quartz is chemically inactive.
Accessory Minerals:
An accessory mineral is that mineral present in the parent rock in such a small quantities that its presence or absence in that rock does not make any difference in the nomenclature of the concerned rock.
A few most common and important accessory minerals are as follows:
(i) Calcite—CaCO3
(ii) Dolomite—CaMg (CO3)2
(iii) Gypsum—CaSO4.2H2O
(iv) Magnetite—MgCO3
(v) Magnetite—Fe3O4
(vi) Chlorapatite—[Ca3(PO4)2]3CaCl2
(vii) Flour apatite—[Ca3(PO4)2]3.CaF2
(viii) Carbonate Apatite—[Ca3(PO4)2]3.CaCO3
(ix) Oxypatite—[Ca3(PO4)2]3CaO
Fundamental Concepts of Identification of Minerals:
Minerals are identified on the basis of their physical properties, and chemical composition. They are also examined as thin polished sections under the petrographic microscope.
The following characters of minerals are noted in the laboratory:
1. Nature of the mineral crystal:
A crystal is a homogeneous body bound by smooth plane surface. A symmetry line or axis of a crystal is an imaginary line through a crystal, around which the crystal may be rotated when two or more identical views of the crystal are seen in one rotation.
Crystals of a mineral may be one of the following three forms:
(i) Euhedral crystals with perfectly developed faces;
(ii) Subhedral crystals with imperfectly developed faces, and
(iii) Anhedral crystals with no developed faces.
The axes of the crystal may be either of the following:
(i) Isometric or cubic crystal with three mutually perpendicular axes of equal length.
(ii) Hexagonal crystal with four axes, three of which are of equal length intersecting each other at 120° angle, with a fourth axis of different length, perpendicular to the plane of the first three axes.
(iii) Tetragonal crystals with three mutually perpendicular axes, two horizontal axes of equal length and the vertical axis of different lengths.
(iv) Orthorhombic crystals with three mutually perpendicular axes of different lengths.
(v) Triclinic crystals with three axes of different lengths. They are inclined at an angle with respect to one another.
(vi) Monoclinic crystals with three axes of different lengths. One of them is incline a with respect to the other two.
2. Form:
The degree of the development of the crystals of the minerals may be as follows:
(i) Crystallized minerals with well-formed big crystals of a regular shape.
(ii) Crystalline minerals lacking an outward regular form although they possess a distinct internal molecular structure.
(iii) Crypto-crystalline where the crystals of the mineral can only be seen in the thin section under the microscope.
(iv) Amorphous: Crystals are totally undeveloped.
3. Specific Gravity of a mineral is the ratio of the weight of a given volume of the mineral to the weight of an equal volume of water.
4. Cleavage of minerals is the property of breaking along definite planes as described below:
(i) Perfect or distinct cleavage where the broken surface is very smooth.
(ii) Imperfect or indistinct cleavage where the broken surface is not smooth.
5. The hardness of minerals is a measure of its ability to scratch another mineral, which has been placed lower in the scale of hardness than the concerned mineral.
Mohs’ scale of hardness of different is as follows:
(i) Talc
(ii) Gypsum
(iii) Calcite
(iv) Fluorite
(v) Apatite
(vi) Feldspar
(vii) Quartz
(viii) Topaz
(ix) Corundum
(x) Diamond
Only the fresh surface of the mineral should be used for scratching.
6. The luster of a mineral is the appearance of its surface in the reflected light as described below:
(i) Adamantine: The surface is like that of a diamond.
(ii) Vitreous: The surface is like that of a glass.
(iii) Resinous: The surface is like that of the yellow resin.
(iv) Greasy: The surface is like that of the grease.
(v) Pearly: The surface is like that of the pearl.
(vi) Silky: The surface is like that of the silk.
7. Colour:
Minerals may be different colours.
8. Streak:
The mineral is rubbed against an unglazed porcelain plate. The colour of the powder of the mineral that is obtained by rubbing it against the unglazed porcelain plate is known as the streak of the mineral.
9. Fracture is the nature of the surface produced when the mineral breaks in a direction other than the cleavage plane.
The broken pieces may exhibit:
(i) Concave surface when the fracture is called conchoidal.
(ii) Irregular surface when the fracture is called uneven.
(iii) Somewhat smooth surface when the fracture is called even.
(iv) Very much irregular surface when the fracture is called hackly
(v) Splinter like parts when the fracture is called splintery.
10. Twining occurs when the crystals of the mineral have grown together or they penetrated each other.
11. Miscellaneous physical properties:
A few minerals may be identified by their magnetic properties. Some may flash different colours whenever they are turned. Some have the property of fluorescence, which means they emit light from within when exposed to ultra violet light. Some may be identified by their taste.
Description and Composition of Rock/Soil Forming Minerals:
1. Pyroxene:
The pyroxene group includes:
(i) Enstatite,
(ii) Hypersthene,
(iii) Augite,
(iv) Diopside and
(v) Rhodonite.
Enstatite has orthorhombic crystal. Augite and Diopside have monoclinic crystals. Rhodonite has triclinic crystals. They may be of massive (heavy or bulky) lamellar (thin leaf like sheets) or granular forms. They have two sets of cleavages, nearly at right angles to each other, and an uneven fracture. They are silicates of calcium, magnesium, iron, aluminum and sodium. The formula of Augite is (CaNa) (MgFeAITi) AL2O6.
Its colour varies from dark green to black. Its streak is whitish grey. It is of vitreous lustre. Its hardness is six. Its density varies from 3.2 to 3.5. Enstatite (MgSi2O6) is of light green colour, white streak and vitreous lustre. Its density varies from 3.2 to 3.9.
2. Amphibole:
The amphibole group includes Hornblende, Actinolite, Tremolite, Anthophyllite and Aenigmatite. Hornblende, Actinolite and Tremolite possesses a monoclinic crystal system and Aenigmatite possesses a triclinic crystal system. They are usually massive (bulky and heavy) but may also be fibrous or granular.
These minerals have a perfect cleavage (two sets inclined at angles 124° and 56°) and an uneven fracture of black, green or greyish colour. The Hardness is 5 to 6 and specific gravity is 2.9 to 3.5. They are similar to pyroxenes in composition by they contain a little water. Formula of Hornblende is Ca(MgFe)4 Al(SiAl) O22(OH).
3. Feldspars:
Feldspars include potash feldspars or orthoclase and microcline and soda-lime feldspars or plagioclase feldspar which includes sodium feldspar or albite and calcium feldspar or anorthite.
(i) Orthoclase and microcline:
Orthoclase possesses monoclinic crystals and microcline processes a triclinic crystal system. The colour of orthoclase is white, pink or gray. Microcline is cream green coloured. Both are massive (bulky and heavy) in form and have an uneven fracture, and are un-coloured. The lustre of both is vitreous. The specific gravity and the hardness of both are 6 and 2.55 respectively. Their formula is KAlSi3Os.
(ii) Plagioclase feldspars are all twined:
Albite (NaAISi3O8), labradorite or andesine NaCa (AlSi2O8) and ahorthite (CaAl2Si3Os) belong to plagioclase Feld spar. They are of triclinic crystal system, massive (bulky and heavy) form, perfect cleavage (two sets are nearly at right angles to each other) uneven fracture, un-coloured streak and vitreous lustre 6 to 6.5 hardness and of specific gravity of 2.60 to 2.76.
4. Mica:
Mica includes muscovite and biotite, both of which are of monoclinic crystal system, perfect cleavage, flaky or scaly form and of pearly lustre. Fracture is not obtainable in both the cases due to good cleavage.
(i) Muscovite is colorless, or of grayish or pinkish colour, un-coloured streak, 2 to 2.25 hardness, with a specific gravity varying from 2.7 to 3.0. It is of pearly to vitreous lustre. Its formula is K2,(LiAl2)(Al4)O20(OH)4.
(ii) Biotite is black or dark green in colour, with an un-coloured streak. It is often grayish or brownish due to decomposition.
Hardness is 2.5 to 3.0. It specific gravity varies from 2.7 to 3.1. The formula K(Si6AI2)(MgFe)6O20(OH)4. Both muscovite and biotite are transparent but the former is of pearly to vitreous lustre whereas the latter is of vitreous to a pearly lustre.
5. Olivine:
It is green or yellowish green is colour, yet lacks colour in the streak. It is of massive form, as an imperfect cleavage, conchoidal fracture and vitreous lusture, with a hardness of 6.5 to 7.0 and specific gravity of 3 to 3.4. The formula is (MgFe)2SiO4.
6. Calcite:
Calcite commonly occurs as hexagonal crystals but sometimes occurs in fibrous, granular or nodular (aggregate of big rounded bodies) form. White or colorless calcite has a perfect cleavage, vitreous lustre, the hardness is 3 the specific gravity 2.7 and the formula CaCO3.
7. Dolomite:
It has a hexagonal crystal system and granular form, uneven to conchoidal fracture. It is of usually white or sometimes pinkish and has a white streak. The hardness is 3.5 to 4.0 the specific gravity is 2.8 and the formula is CaCO3.MgCO3.
8. Gypsum:
It usually occurs in the monoclinic crystal form, but may also occur in the granular, fibrous or massive form. It has a perfect cleavage and uneven fracture. It is white greyish white or pinkish white colour, white streak and vitreous lustre. The hardness and the specific gravity of gypsum are 2 and 2.3 respectively. The formula is CaSO4.2H2O.
9. Kaolin or Kaolinite:
Monoclinic crystals have a perfect cleavage, and uneven fracture. It easily breaks down to powder. It is of white, greyish white or brownish white colour, with a white streak and dull lustre, the specific gravity is 3.2 and the hardness is 5.
10. Tourmaline:
It occurs in the prismatic form. Hexagonal crystals are striated length wise. It has an imperfect cleavage, uneven to somewhat conchoidal. It is black, green, red or brown has an un-coloured streak and a vitreous lustre. The hardness and specifies gravities are 7.5 and 3.0 respectively. It is complex borosilicate of aluminum with a little magnesium, iron and alkali metals.
11. Garnet:
Isometric crystals of garnet occur in the granular form. They are very heavy with a conchoidal fracture and no cleavage. They are black or green and have an un-coloured streak and vitreous lustre. The hardness and the specific gravity are 7.0 and from 3.2 to 4.3 respectively. Formula is (CaFeMgAl) SiO4.
12. Bauxite:
It is amorphous and occurs in the granular, earthy, concretionary, colitic (aggregate of bodies resembling fish roe) or pisolitic (aggregate of pea like masses from with no cleavage and sub-conchoidal fracture. It is greyish white or brownish white in colour with a dull or earthy lustre. The hardness and the specific gravities are 2 and 2.5 respectively. The formula is Al2O3.2H2O.
13. Chlorite:
It is micaceous in appearance due to its perfect cleavage. The crystal are hexagonal tabular and often bent. Green chlorite occurs as a scaly coating on other minerals. The formula is Mg6(OH)12 (Si6Al2) (Mg3Fe3) O20(OH)4.
14. Serpentine:
It occurs as fine granular masses sometimes fibrous. It is dark green and has greasy, waxy or silky lustre and hardness of 2.5 to 3.0. The formula is Mg6 (OH)8Si4O10.
15. Talc:
It is pearly, greasy or waxy lustre and soapy or greasy feel. It’s of white, sea green or grey colour. The formula Mg3(OH)2Si4O10.
16. Chalcopyrite:
Tetragonal crystal of chalcopyrite commonly occurs in the massive form with indistinct cleavage and uneven fracture. It is of golden yellow colour, has a greenish black streak and metallic lustre. The hardness and the specific gravity are 3.5 to 4 and 4.2 respectively. The formula is CuFeS2
17. Malachite:
Crystals of Malachite are very rare. It usually occurs in massive, botryoidal (aggregate of grape like masses) fibrous or encrusting form, with an indistinct cleavage and conchoidal fracture. It is green, with a pale green streak, an often silky or otherwise dull lustre. The hardness is 3.5 to 4.0, the specific gravity is 4.0 and the formula is CuC03.C.a (OH)2
18. Azurite:
Monoclinic crystals of azurite are very fare. It is usually of massive and earthy form with an indistinct cleavage and conchoidal fracture, deep sky blue colour and pale blue streak and vitreous to adamantive lustre. The hardness is 3.5 to 4. The specific gravity is 3.8. The formula 2CuCO3Cu (OH)2.
19. Pyrite:
Brass yellow coloured pyrite occurs in striated cubic crystals, massive, fibrous, re-inform (kidney-shaped) or granular form. The crystal system is isomeric. It has greenish black streak, metallic lustre 6 to 6.5, and hardness and specific gravity are both 5. The formula is FeS2.
20. Hematite:
Hexagonal crystals of hematite commonly occur in the tabular form. It also occurs in re-inform, botryoidal, scally or foliaceous form. It has an indistinct cleavage and uneven fracture. It is of steel and metallic lustre. Its hardness varies from 5.5 to 6.5 while its specific gravity is 5.2. The formula is Fe2G3.
21. Limonite:
Amorphous limonite occurs in mammillary (aggregate of masses bigger than that of grapes) or botryoidal (aggregate of grape like masses or fibrous or concretionary or earthly or massive form. The cleavage is absent but the fracture is uneven. It is brown, has a yellowish brown streak and dull or silky lustre. The hardness and the specific gravity are 5.5 and 4.0 respectively- The formula is 2Fe2O3.3H2O
22. Magnetite:
Isomeric crystals of magnetite are usually or octahedral form, but they may also occur in massive or granular form. It is magnetic with an indistinct cleavage and uneven fracture. Colour and streak and both black iron. It has a metallic to dull lustre. The hardness is 5.5 to 6.5 and the specific gravity is 5.1. The formula is Fe3O4
23. Magnesite:
It is a hexagonal crystal system and massive compact or earth form with perfect cleavage and conchoidal fracture colour is white or greyish white or brownish white. Streak is white. It is white. It is of vitreous 4.0 to 4.5 hardness and a specific gravity of 3. The formula is MgCO3.
24. Ilmenite:
A hexagonal crystal system of ilemenite occur tabular massive, compact, granular or loose form as sand with indistinct cleavage and conchoidal fracture. It is of iron black colour, black or brownish black streak and sub- metallic lustre. The hardness and the specific gravity varies from 5 to 6 and from 4.5 to 5.0 respectively.
25. Montmorillonite is greyish yellow in colour, with a white streak and dull lustre. Its hardness varies from 2.0 to 2.5 and its specific gravity varies from 2.0 to 2.7.
26. Glauconitic is of green to brown in colour, greenish grey in streak, earthy to dull lustre. It is opaque. Its hardness and specific gravity are about 2.5 and 2.65 respectively.