ADVERTISEMENTS:
After reading this article you will learn about the physical and chemical processes of weathering rocks and minerals.
1. Physical or Mechanical Weathering:
ADVERTISEMENTS:
The physical or mechanical weathering process may be defined as the process by which disruption of consolidated massive rocks into smaller bits was found without any corresponding chemical change or formation of new products.
Under extreme climatic conditions like very cold and hot conditions, the physical weathering is more prominent. Physical weathering, a slow process, causes changes only in size, thus accelerating chemical reactions by exposing more reacting surfaces as the size of rocks and minerals becomes smaller.
The physical condition of rocks is probably the most important single factor which determines the rate at which the rocks are subjected to weather. For an example, a coarse textured (porous) sandstone will weather more easily and rapidly than that of fine textured (almost solid) basalt because of the greater permeability.
Temperature and water are the most important agents of physical weathering. Due to changes in temperature in different seasons of the year, the expansion and contraction in surface layers of rocks was found resulting breaking down of bigger size rocks. The changes of temperature also bring about exfoliation of the surface layers of rocks.
ADVERTISEMENTS:
Water causes physical weathering of rocks in many ways. Water acts as disintegrating, transporting and depositing agent. The moving water has got a tremendous cutting and carrying force. When water freezes in rocks joints and crevices, it expands to about 9 per cent of its volume, creating a shattering force of about 150 tons per square foot. This is a hydrothermal process caused by intermittent freezing and thawing and is more predominant in temperate regions.
Frost is much more effective than heat in performing physical weathering as mentioned above. Besides, some natural substances increase considerably in volume during wetting and shrink on drying. The effect of alternate wetting and drying on clay-enriched rocks with platy structure, especially shale, is to make them loose and eventually the rocks break.
The action of glaciers is also very important in carrying out the physical weathering. In temperate regions, when snow falls, it accumulates and turns into ice. The big glaciers start moving due to change in temperature and slope gradient. During movement, the glaciers exert a tremendous pressure on rocks and grind the rocks. The movement of glaciers stops when it starts melting due to rise in temperature elsewhere.
Wind, especially when laden with sand particles, cause abrasive action on exposed rocks. Wind in combination with ocean waves causes weathering along the coastal areas. Atmospheric electrical phenomenon is also an another factor which causes physical weathering. During rainy season, the frequent lightening occurs and during lightening time rocks break up and wider cracks form.
2. Chemical Weathering:
ADVERTISEMENTS:
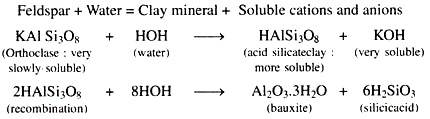
The process of chemical weathering may be defined as the transformation of original rocks and minerals into new compounds having different chemical composition and physical properties. As for an example, the chemical weathering of feldspar produces a clay mineral of different composition and physical properties.
Feldspar + Water → Clay mineral + Soluble cations and anions
No chemical weathering is possible without the presence of water. Chemical weathering becomes more effective as the surface area of the rock increases since the chemical reactions take place mostly on the surface of rocks. It is the most important process so far as soil formation is concerned.
The effectiveness of chemical weathering is very closely related to the mineralogical composition of rocks. As for example, a mineral like quartz (SiO2) responds very slowly to chemical attack than that of a mineral like olivine (Fe, Mg)2SiO4 because of their differential weather ability.
ADVERTISEMENTS:
However, the rate of chemical reaction increases with dissolved CO2 and other solvents in water, and with increases in temperature. So in desert areas due to lack of water and in temperate regions due to low temperature, the chemical weathering is minimum, whereas maximum chemical weathering occurs in tropics where both water and temperature conditions are more conducive. The plants, animals and other organisms also contribute directly or indirectly to chemical weathering since they produce CO2, O2 and other certain acids that react with rock materials.
The various important agents of chemical weathering are described below:
(i) Solution:
Solution, the dissolving of a solid in a liquid, separates solid materials into independent soluble ions, each surrounded by the liquid molecules. Solution permits greater chemical changes than that of an un-ionized (usually solid) state.
ADVERTISEMENTS:
Water is a universal solvent. Most of the minerals are affected by the solvent action of water. Solution helps in a continual loss of weathered material but total removal by simple solution is negligible.
Solution is represented by the following typical equation of a salt dissolving in water:
NaCl (A soluble salt) + H2O(Water) → Na+, CI–, H2O(Dissolved ions, surrounded by water molecules)
The action of solution process is considerably increased when the water is acidified by the dissolution of organic and inorganic acids.
(ii) Hydrolysis:
Hydrolysis, which involves the splitting of water into H-ions and OH-ions, is the reaction of substances with water to form hydroxides and other new substances that are usually more soluble than the original material.
Hydrolysis is conveniently brought about as follows:
Hydrolysis is a double decomposition process. All other silicates are also changed in a similar way.
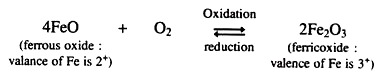
(iii) Oxidation-Reduction:
Oxidation, as referred to in mineral weathering is both the chemical combining of oxygen with a compound and the loss of electrons (change in oxidation number) of some chemical element. Oxidized minerals have a volume increase with added oxygen and are usually softer than the un-oxidized material. If the oxidation number of an element is changed, this can also imbalance the electrical neutrality of the mineral, making it more easily attacked by water and carbonic acid (H2CO3).
Reduction is the chemical process in which electrons are gained, the negative charge is increased, and the positive charge is decreased. In soils, reduction usually occurs when oxygen is scarce, as in stagnant water conditions. Reduction in minerals may result in electrically unstable compounds, in more soluble ones, or in changes in atom size causing internally stressed conditions. All these eventually cause faster decomposition.
The importance of oxidation-reduction in soil formation is that it can speed mineral breakdown by making some minerals more soluble. Oxidized ions are smaller and higher charged than the ions in their reduced state, so minerals are unstable and weather very rapidly as some of their atoms oxidize. Oxidation and reduction are always companion processes.
(iv) Hydration:
Hydration is the chemical combination of a solid substance viz. a mineral or a salt the water. Hydration water combining within the mineral changes the mineral structure, increasing its volume due to swelling and thereby making it softer, more stressed, and more easily decomposed.
Under desiccating conditions, the water of crystallization is given out and the mineral formed due to hydration is converted to its original form.
(v) Carbonation and other acidic processes:
Carbonation is the combination of carbon dioxide with any base. This process is very much effective of the other chemical weathering processes. The presence of H+ ions in percolating waters and other inorganic acids like HNO3, H2SO4 and some organic acids accelerate the decomposition of the minerals through chemical weathering.
The carbon dioxide gas readily combines with bases to produce carbonates and bicarbonates as:
CO2 + 2KOH → K2CO3 + H2O
K2CO3 + H2O + CO2→ 2KHCO3
Carbonic acid (H2CO3) dissolves minerals more readily than does water alone and forms the soluble bicarbonates as follows:
CO2 + H2O → H+ + HCO3
CaCO3 (slightly soluble) + H + HCO3→ Ca(HCO3)2(readily soluble)
Besides carbonation, the reaction of hydrogen ions with soil minerals is that of an acid clay with a feldspar like anorthite:
Due to recrystallization process clay is produced from the so formed acid silicates. So from the various processes of chemical weathering the main products of it are very small (less than 2µ) which carry a significant negative electric charge on their surface and have mica-like structures, the most common type of which is hydrous mica.