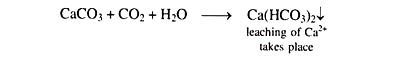ADVERTISEMENTS:
After reading this article you will learn about:- 1. Forms of Calcium (Ca) 2. Sources and Distribution of Ca 3. Behaviour 4. Leaching Losses 5. Plant Factors 6. Deficiency and Disorders 7. Fertilizers Containing Calcium.
Forms of Calcium (Ca):
1. Forms Taken up by Plants:
Calcium is absorbed by plants as its Ca2+ ion. However, such absorption takes place from the soil solution and probably by root interception or contact exchange. Mostly, calcium can be readily transported to root surfaces by mass flow process, excepting in highly leached and un-limed soils.
In soils of temperate region, calcium content varies from 30 to 900 mg kg-1, whereas in soils of high rainfall areas, the concentration of Ca in the soil solution varies from 8 to 45 mg kg-1.
ADVERTISEMENTS:
Calcium concentration in the soil solution is present about 10 times greater than that of K concentration. In-spite of presence of greater concentration of Ca in the soil solution, its uptake by plants is lower than that of K as the capacity of the uptake of Ca is limited to only by young root tips.
2. Forms of Ca in Soils:
Calcium is present in soils as its various forms viz. mineral particles, CaCO3, simple salts and exchangeable Ca.
(i) Mineral particles:
Calcium is mainly present as primary minerals such as basic plagioclase rich in anorthite, epidote, basalt, diabase. On weathering all these minerals release Ca in the soil.
ADVERTISEMENTS:
(ii) CaCO3:
The Nodular form of CaCO3 and even amorphous CaCO3 exist in the soil.
(iii) Salts:
Calcium is also present as its simple salts like CaCl2, CaSO4, CaNO3, Ca(HCO3)2 etc. in soils.
ADVERTISEMENTS:
(iv) Exchangeable Ca:
Among various cations, Ca is the dominant exchangeable cation present on the soil exchange complex.
Sources and Distribution of Ca:
The mean Ca content of the earth’s crust is about 3.64%. It is usually far greater amount than that of most other plant nutrients. Calcium is present in soils in various primary minerals, which include Ca-bearing Al-silicates namely feldspars and amphiboles, Ca-phosphates and Ca-carbonates.
The Ca content of different soil types varies widely depending upon the nature of parent materials and also with the intensity of weathering. The weathering of primary Ca-bearing minerals depends upon the formation of H+ in the soil which causes dissolution of the mineral with the subsequent release of Ca.
ADVERTISEMENTS:
In soils containing a substantial amount of free CaCO3, the formation of Ca(HCO3)2 takes place in presence of sufficient amount of CO2 in the soil as follows:
In addition, the formation of NO3– appears to play an important role in balancing Ca2+ and also Mg2+ in the leaching process.
Under natural conditions NO originates largely from soil organic matter by mineralization and subsequent nitrification as follows:
ADVERTISEMENTS:
2 NH4+ + 4O2→ 2 NO3– + 4H+ + 2H2O
Hydrogen ions so produced can release Ca2+ by exchange from soil colloids. The rate of production of H+ ions from the above nitrification process is greater and therefore, it exerts a major influence on soil acidification and the leaching of Ca.
Amount of Ca content in soils varies from soil to soil and also variations in climatic conditions. The concentration of Ca in CaCO3 free soils of humid temperate regions usually ranges from 0.7-1.5%. Calcium content in highly weathered soils of humid tropics and calcareous soils varies from 0.1-0.3 and < 1 to >25% respectively. However, coarse textured soils formed from rocks low in Ca-containing minerals and line textured soils formed from rocks high in Ca-containing minerals contain low and high amounts of Ca respectively.
Behaviour of Ca in Soils:
In acid soils of humid regions, Ca mostly occurs in the exchangeable form and as un-decomposed primary minerals.
The exchangeable and solution forms of Ca are in dynamic equilibrium as follows:
If the activity of Ca2+ in the soil solution phase is decreased, there tends to be replacement from the adsorbed phase. On the other hand, if the activity of Ca2+ in the soil solution is increased (due to addition of Ca2+ in the soil), there tends to be a shift of equilibrium in the opposite direction, with the subsequent adsorption of some of the Ca2+ by the soil exchange complex.
The availability of Ca in soils has been found to be affected by the following soils factors:
(i) Supply of Ca
(ii) Soil reaction or pH
(iii) Cation exchange capacity (CEC) of soils
(iv) Per cent saturation of Ca in soil colloids
(v) Nature, amount and type of soil colloids
(vi) Ratio of Ca to other cations in soils
(i) Supply of Ca:
In sandy soils having very low CEC, the amount of Ca in soils is too low to provide Ca for the growth and nutrition of crops.
Therefore, in such soils the application of Ca is necessary to supply Ca to crops—as well as to correct soil acidity.
(ii) Soil Reaction or pH:
Soils having acidic in reaction or containing high amount of H+ ions, very frequently reduce the availability of Ca in soils and hence uptake by crops.
(iii) Cation Exchange Capacity (CEC):
A soil having only 1000 kg of exchangeable Ca per hectare but exhibiting a high degree of Ca saturation of a low CEC may supply Ca to a greater extent to plants than that of a soil containing 5000 kg of exchangeable Ca per hectare but with low saturation of Ca of a higher CEC. Therefore, the value of CEC is not of so important, rather the degree of Ca saturation in soil colloids is very important to supply Ca to plants.
(iv) Per cent Saturation of Ca in Soil Colloids:
It is evident that many crops show a response to the applications of Ca in soils where the degree of Ca saturation of the total CEC falls below 25%. High Ca saturation is an indicative of a favourable soil reaction or pH for the growth of most plants as well as microbial activity. Calcium saturation at < 40-60% and Al saturations of 40-60% lower the yield of crops especially cotton.
(v) Nature, Amount and Type of Soil Colloids:
The type of soil colloids particularly clay colloids influence the magnitude of Ca availability, for an example, 2: 1 type clays require a higher degree of Ca saturation for a given level of plant utilization than that of 1 : 1 type of clays. Montmorillonitic clays (2:1) require a Ca saturation of 70% or more before the release of Ca in soils and subsequently available to growing plants.
Kaolinitic clay (1: 1), however, is able to supply Ca to plants at saturation values of only 40-50%. The amount of exchangeable Al rather than the per cent saturation of Ca was more important in determining the amount Of Al in the soil solution of organic soils.
(vi) Ratio of Ca to other Cations in Soils:
The availability of Ca and its uptake by plants are also affected by the ratios of Ca to other cations in soil solution. However, a Ca: total cation ratio of 0.10-0.20 is beneficial for the growth and development of most crops. Blossom-end rot (Ca-deficiency disorder) in tomatoes can be prevented by maintaining Ca: total cation ratios in the range of 0.16 to 0.20.
The uptake of Ca has been found to be reduced in soils containing a sufficiently higher amount of NH4+—N, K+, Mg2+, Mn2+ and Al3+. However, the absorption of Ca by plants increases in soils supplied with NO3—N.
The exchangeable Ca is of particular importance in soil aggregation and hence for soil structure. Calcium promotes flocculation of soil colloids and improves soil structure and the stability of soil particles. The adsorption sites of the inorganic soil colloids are not very selective for Ca2+.
As the electrostatic charge of Ca2+ is high due to its di-valency and rather thin hydration shell, Ca is relatively strongly adsorbed to various types of clay minerals in the soil. The adsorption bond of Ca2+ to organic colloids and especially to the humic acids is more specific. Calcium adsorbed onto soil colloids tends to equilibrate with the Ca2+ of the soil solution.
Deficiency symptoms of Ca on crops is of rare occurrence as most soils contain high amounts of Ca in the soil solution as well as in the exchange sites of soils. In addition, there are some other parameters like leaching losses of Ca and plant factors which can affect the availability of Ca in soils vis-a-vis to plants.
Leaching Losses of Ca:
It is one of the ways for the losses of Ca in soils. Despite leaching, Ca is also lost from soils by crop removal and losses due to conversion of soluble Ca into insoluble form of Ca in soils.
However, the loss of Ca in drainage water due to leaching resulting from the heavy rainfall or through the interaction of fertilizers and manures occurs very frequently. Such loss increases with rainfall. Light textured soils suffer a greater loss of Ca than that of heavy textured soils.
Besides, the use of certain fertilizers viz. (NH4)2SO4 and NH4Cl encourage the loss of Ca with the simultaneous increase in soil acidity. Losses of Ca through leaching depend on the amount of Ca present in the soil, amount of rainfall, and texture of the soil etc. Calcium is often the dominant cation in drainage waters, lakes, streams etc. Leaching losses of Ca vary on an average from 75 to 200 kg ha-1 per year.
Plant Factors of Ca:
Conditions restricting the growth of new roots reduce access of plant roots to Ca and hence induce Ca deficiency on plants. Plants having poor or small root systems exhibit very poor uptake of Ca by plants causing Ca deficiency. During evolution plant species have adapted to varying pH and Ca conditions. For this reason wide differences in toleration of Ca occur between plant species and even within plant species.
On that basis, plant species may be divided into Calcicoles (Ca loving slants)—plants grow on calcareous soils containing sufficient amount of Ca and Calcifuges (Ca-sensitive plants)—plants grow on acid soils containing very low amounts of Ca or soils poor in Ca.
Many of the calcicole plant species contain high amounts of intracellular Ca and high concentrations of malate, whereas, the calcifuges are usually low in soluble Ca. Plants may differ considerably in their capability to precipitate Ca as Ca-oxalate.
Another important aspect in the calcicole—calcifuge problem is the ability of different ecotypes to utilize Fe. Plant species originating from acid soils are more susceptible to lime- induced chlorosis (a physiological disorder—characterised by a light green to yellow colour of the youngest leaves resulting from the excess of HCO3–in calcareous soils).
The susceptibility of the ecotypes from acid soils to lime-induced chlorosis was not due to the lack of Fe-uptake by roots rather due to inability of roots to metabolize Fe. Lime induced chlorosis can often be a serious problem to grow crops on calcareous soils.
Deficiency of Ca and Its Disorders:
Deficiency of Ca is characterised by a reduction in growth of meristematic tissues. The deficiency of Ca can be first appeared in the growing tips and youngest leaves, and subsequently at a more advanced stage necrosis occurs at the leaf margins.
The affected tissues become soft due to a dissolution of the cell walls. Brown substances occur which accumulate in intracellular spaces and also in the vascular tissue where they can affect the transport mechanism.
Although, the deficiency of Ca does not occur as most mineral soils rich in available Ca. Indirect Ca deficiency arising from an undersupply of Ca to fruits and storage tissues oftenly occurs.
In tomato, the disease is known as blossom-end rot and is characterised by a cellular breakdown at the distal end of the fruit. An identical Ca-deficiency disorder occurs in watermelon, in vegetables such as black heart of celery, and cavity spot of carrots.
Calcium appears to be transported from the soil solution to the upper plant parts via root tips. Any factor which prevents the growth of new roots (poor aeration, low temperature etc.) may be expected to prevent Ca uptake and thus induce deficiency. However, the deficiency of Ca if occurs, may be met up by the application of Ca- containing fertilizers to soils.
Fertilizers Containing Calcium:
We know that the occurrence of Ca deficiency is very rare. Therefore, Ca is not usually formulated as such into mixed fertilizers but rather it is present as a component of the materials supplying other nutrients.
However, some Ca-containing materials are listed follow:
Name of the material containing Ca-Ca-content (%)
Single Super Phosphate – 18-21
Tripple Super phosphate – 12-14
Calcium nitrate [Ca(NO3)2] – 19
Ca-EDTA (Synthetic chelate) – 3-5
Gypsum (CaSO4.2H2O) – 23