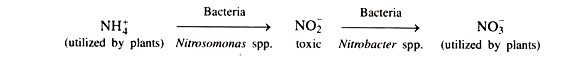ADVERTISEMENTS:
A project report on soil pollution. This project report will help you to learn about: 1. Introduction to Soil Pollution 2. Definition of Soil Pollution 3. Kinds 4. Causes 5. Sources 6. Effects 7. Diseases 8. Controls.
Contents:
- Project Report on Introduction to Soil Pollution
- Project Report on the Definition of Soil Pollution
- Project Report on the Kinds of Soil Pollution
- Project Report on the Causes of Soil Pollution
- Project Report on the Sources of Soil Pollution
- Project Report on the Effects of Soil Pollution
- Project Report on the Diseases Caused by Soil Pollution
- Project Report on the Controls of Soil Pollution
Project Report # 1. Introduction to
Soil Pollution:
ADVERTISEMENTS:
Industry being a voracious consumer of natural resources brought in pollution of air, water and soil environment. Soil pollution usually originates from the development of industry, intensive agriculture associated with modern systems of cultivation like use of high analysis chemical fertilizers, use of pesticides, use of sewage, sludge, city composts and other industrial wastes etc.
The contaminants produced by industrial processes ‘reach the soil by three ways:
(a) Through the air—gaseous and particulate contaminants are emitted from chimneys and exhausts or are deflated from spoil heaps and are blown away by the wind ultimately to sink or be washed by rain to the soil environment.
(b) Through drainage system industrial effluents and water drainage from spoil and rubbish heaps either washes direct on to nearby fields or enters the local streams and rivers and ultimately enter into the soil, and
ADVERTISEMENTS:
(c) Through direct mechanical or gravitational effects on the soil-solid materials like refuses from mines, animal slurries or sewage-sludge’s etc. can be dumped directly on to the agricultural-land and thereby creates soil pollution.
Once pollutants enter and are incorporated into the soil, their concentrations in soil are continuously increasing and accumulating as toxic to all forms of life like plants, microorganisms, human beings etc.
Project Report # 2. Definition of Soil Pollution:
The word “soil” have been derived from lain word “solum” meaning upper crust of the earth. Soil is actually formed as a result of long term process of complex interaction leading to the production of a mineral matrix in close association with interstitial organic matter — living as well as dead. Soil is one of the most important ecological factors. Soil is thus usually defined as “any part of earth’s crust in which plant root”.
ADVERTISEMENTS:
Soil pollution is the reduction in the productivity of soil due to the presence of soil pollutants. Soil pollutants have an adverse effect on the physical, chemical and biological properties of the soil and reduce its productivity.
Project Report # 3. Kinds of Soil Pollution:
Soil gets polluted by a number of ways. The major kinds of soil pollution are:
i. Acidification:
The causes of acidification are both natural and anthropogenic as shown below.
(a) Natural causes:
The natural causes include the following:
(i) Long-term leaching:
ADVERTISEMENTS:
Acids found in rainwater (carbonic acid) and in the decomposition of organic material (humic and fulvic acids), can stimulate leaching by dissociating into H+ ions and their component anions then displace or attract base cations from the soil exchange complex.
(ii) Microbial respiration:
During microbial respiration soil acidification takes place through the production of CO2, which dissolves in soil water to form carbonic acid.
(iii) Plant growth:
During plant growth nutrient base cations are obtained through root systems in exchange for H+ ions, thus leading to increased soil acidity.
(iv) Nitrification:
It is an oxidative process of organic decomposition where ammonium (NHJ) ions are converted to nitrate (NO3) ions by nitrifying bacteria, with H+ ions as a byproduct:
NH+4 + 1.5O2 → NO−3 + 4H+
These ions then displace and attract base cations from the soil exchange complex, thus leading to soil nitrification.
(b) Anthropogenic causes:
The main anthropogenic causes of acidification include certain land-use practices such as:
(i) Needle-leaf afforestation:
Needle-leaf afforestation has been associated with acidification of soils for the following reasons:
1. Needle-leaf trees produce litter which is very acidic in comparison with other broadleaf species
2. Due to their high canopy surface area, needle-leaf trees are able to scavenge acid pollutants from the atmosphere. These are later released into the soil by through fall and stem flow.
(ii) Excessive use of inorganic fertilizers:
This also causes acidification partly through the process of nitrification (as is given above). If levels of NO3 ions in the soil are in excess of plant requirements, they will behave as mobile anions and thus encourages leaching processes. If mineral acids predominate, then aluminium is mobilised in its ionic, labile-monomeric form (Al3+).
This form of aluminium is particularly toxic to many freshwater organisms including fish. In a similar way, other heavy metals such as zinc, lead and cadmium, are more readily mobilised in acidic soils. Other causes of acidification include acid deposition due to acid rain resulting from automobile and industrial pollution. Land drainage of acids from industries also adds to the acidification of land.
Effects:
Soil acidification and associated nutrient leaching leads to the damage of trees in forested areas. This includes needle discolouration, crown defoliation and deformed branched structures. Increased soil acidity and associated changes in aluminium mobility also have serious implications on the quality of surface waters which receive drainage from acidified soils.
Buffering or acid neutralizing capacity:
Soils vary in their ability to buffer acidity. Soils which have significant quantities of base-rich, weatherable minerals have a high buffering capacity, while those which are dominated by quartz and similar resistant minerals have a low buffering capacity.
Management and Remediation:
Remediation and management of soil and surface water acidity can be done by a number of methods such as liming, sensible forestry management and reduction of acid emission. To rectify acidity in agricultural lands liming has been practiced for a long time. Liming material most commonly used are limestone, chalk and basic slag.
Other minor components used are quicklime (CaO), slaked lime [Ca(OH)2] and magnesium carbonate (MgCO3). The requirement of lime in a soil depends on its buffering capacity. It is usually expressed as the amount of CaCO3 required (in t/ha) to raise the pH of the top 15 cm of soil to the desired value.
In temperate areas, the ideal soil pH is about 6.5 for arable crops and about 6.0 for grasslands. However, the pH value of 5.5 is often preferred in tropical areas, particularly is soils with high exchangeable aluminium contents and where phosphorus availability may be restricted under more alkaline conditions.
ii. Salinisation and Sodification:
Salinisation and sodification may occur naturally in semi-arid and arid environments and also as a result of human activity.
The causes of salinisation and sodification of soil is due to:
(i) Removal of indigenous eucalyptus forest has resulted in salinisation and sodification of soils in south-west Australia due to the replacement of deeply-rooted trees by shallow-rooted grasses and crops. These shallow-rooted grasses are less effective in lowering the ground water level. Salinity and sodicity are greatest in soils where the water table is within about 2 m of the surface,
(ii) Poor irrigational practices also lead to salinity and sodicity. Over watering results in the rise of the water table, which, in turn, causes enhanced capillary actions. Also, poor maintenance of irrigation channels and canals result in leakage of water on to adjacent agricultural lands. Thus increased salinity and sodicity results,
(iii) Construction of dam often results in the deterioration of the soil quality in places which were once very fertile flood plain areas.
The seasonal flood waters (prior to the construction of dams) were particularly important to agriculture for three reasons:
1. They were important source of irrigation water.
2. They brought with them fertile silt deposits.
3. They helped to flush out salts from the soil. Thus, the benefits of seasonal flooding have been removed and the resulting decline in soil quality has been compounded by agricultural intensification in order to meet the needs of a rapidly growing population as well as the demands of more cash crops.
Effects:
Salinity and sodicity have detrimental effects on both physical and chemical aspects of soil.
High salinity and sodicity are associated with:
1. The pH of the soil increases and under such conditions the availability of certain plant nutrients is reduced resulting in severe disturbance of the plant nutrient balance as a whole.
2. Elevated salt and Na+ concentrations in soils are also highly toxic to many plants. However, tolerance level varies from plant to plant. Crops like barley, cotton and sugar beet have a high tolerance level, while low tolerance level is seen in sugarcane, onion and lettuce.
Grasses also vary in their tolerance level to soil salinity and sodicity. For example, Bermuda grasses have a high tolerance level while Guinea grasses are much less tolerant.
Management and remediation:
In case of soil salinity and sodicity, management and remediation are complex issues and may involve a number of approaches. Appropriate irrigation and drainage techniques are to be implemented in saline, saline-sodic and sodic soils.
iii. Agrochemical Pollution:
(a) Fertilisers:
The application of inorganic fertilisers has increased many folds, at the expense of more traditional organic nutrient treatments. In inorganic fertilisers, the nutrients are in a more readily available form and are released rapidly after application. On the other hand, organic material releases its nutrients slowly, through decomposition processes and only when conditions are suitable (not necessarily when crops need them).
Fertilisers are applied either in solution, suspension, emulsion or in solid forms. The solid forms vary from fine powder to coarse granules. They are applied either by evenly spreading over the soil surface or are mechanically placed. Generally the rate of nutrient release decreases with increasing particle size. Fertilisers are based on a variety of nutrient combinations.
Fertiliser applied to the soil undergoes the following fate:
(1) Plant and animal uptake (immobilisation).
(2) Adsorption and exchange in the soil (fixation).
(3) Leaching and loss in soluble form through drainage.
(4) Volatilisation and gaseous loss to the atmosphere (de-nitrification).
(5) Surface loss in solid form by runoff and erosion.
Effects:
Leaching is most common in coarse-textured and well-drained soils. De-nitrification losses are greatest in fine- textured water-logged and poorly aerated soils. Surface losses are greatest at sites which are more susceptible to surface runoff and erosion.
Environmental problems associated with fertiliser use, which has received most attention is nitrate leaching. Levels of nitrate in water supplies have increased particularly where inputs of nitrogen fertilisers have been high.
The main factors which influence the extent of nitrate leaching include land use, soil characteristics and climate. Leaching tends to be greatest when there is no crop cover to utilise the nitrate released from fertilisers or organic reserves.
Management and remediation:
To maximise uptake and minimise leaching, fertilizers should be applied just before and during the period of maximum crop growth. Large applications at any one time should be avoided. Tillage should be practiced as it improves top soil drainage and aeration, which increases the rates of organic matter decomposition.
(b) Pesticides:
A wide range of pesticides are in use today which includes insecticides, herbicide, fungicides and other varieties such as nematicides, miticides, rodenticides and molluscicides. Pesticides may degrade biologically or photo-chemically, ‘ adsorbed by organic matter, clay and oxides/hydroxides of iron and aluminium, washed into water by leaching and surface runoff or they may undergo volatilisation into the atmosphere.
Effects:
Pesticides generally control only the target organisms and then degrade into harmless products. However, some pesticides persist in the environment, cause toxicity in soil, vegetation and water supply and causes impact beyond the target organisms, including bioaccumulation and its implication on human health. Sometimes the degraded products of some pesticides may be as toxic as the original source chemical.
Management and remediation:
Extensive research and monitoring is to be undertaken to minimise effective persistence and toxicity and to maximise specificity Attempts are to be made to model the behaviour of pesticides in soils, with a view to reduce the risk of ground and surface water contamination.
The use of non-chemical strategies of pest, disease and weed control is to be practiced which include direct approaches, biological and cultural methods and habitat removal.
iv. Urban and Industrial Pollution:
Urban and industrial development is associated with both physical degradation and chemical contamination of soils. Physical degradation of soil results from erosion, compaction and structural damage resulting from construction activities and opencast mineral extraction.
Chemical contamination of soil is due to waste disposal activities, discharge and spillage of liquid effluents and atmospheric emissions including acid deposition.
In 1991, Bridges formulated four major sources of soil contamination:
(a) Construction and demolition wastes,
(b) Metalliferous wastes,
(c) Power generation emissions, and
(d) Chemical and organic wastes.
(a) Construction and demolition waste:
These wastes are produced at building sites and consist of broken bricks, tiles, glass, timber, piping, wiring and cables, insulation materials, mortar, pieces of iron rods and nails, asbestos pieces, concrete and plaster.
These materials in soil undergo a number of chemical changes. For example, plaster contains large amounts of gypsum. This gypsum at sites where the water table is high gets dissolved and by capillary actions may bring it into contact with new concrete structures, thus leading to serious corrosion problems.
(b) Metalliferous wastes:
These wastes are made up of heavy metals (such as lead, zinc, cadmium, copper and nickel) and are generally found in soils in areas where ore extraction and smelting have occurred. Metal contamination occurs on land used for scrap metal dealing and for ammunition factories. In soil, toxic metals may exist in a number of forms including adsorbed cations, attached to clay and humus colloids and organo-metallic chelates.
The availability of metals to plants depends upon a number of soil characteristics, such as cation exchange capacity (CEC), pH and the interdependence effects of other metals. In soils with a low CEC, the metals are not retained effectively and are likely to be either leached from the soil or taken up by plants.
In soils with a high CEC, the metals are likely to be fixed in the soil through adsorption processes. Likewise, the mobility and availability of heavy metals is considerably greater in acidic soils than in near neutral or alkaline soils. The metals once mobilised may enter the food chain either through water supplies and aquatic organisms or through the grazing animals.
(c) Power generation emissions:
Power generation industry contaminates the soil by emitting a number of contaminates such as sulphur dioxide (SO2) from coal fired power stations and radionuclides from nuclear power stations and weapon testing. Due to anthropogenic activity the radionuclides commonly found in soils are those of caesium (137Cs and 134Cs).
The behaviour of radionuclides in soils depend upon some soil characteristics, such as clay content and mineralogy, organic content, CEC, pH, NH4 content and nutrient status. A high CEC and near neutral pH values in soil mobilises the radionuclides as they are adsorbed on clay.
In acidic soils and in low CEC soils, radionuclide are least retained and may be available for plant uptake. This ultimately gets incorporated in milk and meat of grazing cattle and sheep.
(d) Organic wastes:
Soil contamination may also take place from a number of organic wastes. Land used for storage and maintenance of motor vehicles and for fuel storage (fuel refilling stations) are often subjected to oil spillage and other related wastes and become badly contaminated by dioxins from waste oils.
Sewage sludge, often applied to agricultural land adjacent to urban and industrial areas, often contains high concentrations of heavy metals. The PCBs (polychlorinated biphenyls) used as dielectric fluids in transformers, are often released into the soil during the break-up of electrical equipment. These are a group of organic solvents commonly implicated in soil contamination.
Project Report # 4. Causes of Soil Pollution:
(a) Direct causes:
Poor waste management
Application of agrochemicals
Family sanitation practice
Salination due to irrigation and flood
Soil erosion
(b) Indirect causes:
Acid rain
Photochemical smog
Radioactive disposed substances.
Project Report # 5. Sources of Soil Pollution:
i. Industrial Wastes.
ii. Urban Wastes.
iii. Radioactive Pollutants.
iv. Agricultural Practices.
v. Chemical and Metallic Pollutants.
vi. Biological Agents.
i. Soil Pollution by Industrial Wastes:
Disposal of industrial waste is the major problem responsible for soil pollution. These industrial pollutants are mainly discharged from pulp and paper mills, chemical industries, oil refineries, sugar factories, tanneries, textiles, steel, distilleries, fertilizers, pesticide industries, coal and mineral mining industries, metal processing industries, drugs, glass, cement, petroleum and engineering industries etc.
It has been estimated that about 50% of the raw materials ultimately become waste products in industry and about 20% of these wastes are extremely deleterious. In United Kingdom, it has been reported that about 20 million tonnes of substances are disposed off in the soil as industrial waste.
With the advent of technology, newer types of industrial wastes are produced and deposited in the land. These waste products are also tipped on soil, enhancing the extent of soil pollution. Thermal, atomic and electric power plants are also the villain to add pollutants to soil.
The furnaces of such industries generate ‘fly ash’ i.e. un-burnt brownish black substance which severely pollute air, water and soil. Many industrial effluents are either discharged into streams or dumped into the surrounding land.
Industrial wastes mainly consist of organic compounds along with inorganic complexes and non-biodegradable materials. These pollutants affect and alter the chemical and biological properties of soil. As a result hazardous chemicals can enter into human food chain, the soil or water, disturb the biochemical process and finally lead to serious effects on living organisms.
Industrial Sludge’s:
Industrial sludge’s are even more dangerous than industrial solid wastes to dispose of tidily. The composition of industrial sludge’s vary enormously, the common boiler scale, for example, consists of calcium carbonate and flue gas sludge.
This flue gas desulphurization sludge (FGDS) is generated when calcium hydroxide or lime stone slurries are used to trap sulphur dioxide from escaping gases in coal fired power plants. These wastes also consist of calcium salts and several toxic volatile elements such as arsenic, selenium, mercury, lead and cadmium, which pose detrimental effects on the environment.
ii. Soil Pollution by Urban Wastes:
Urban wastes comprises both commercial and domestic wastes consisting of dried sludge of sewage. All the urban solid wastes are commonly referred to as ‘refuse’. Soil wastes and refuse, particularly in urban areas contribute to soil pollution.
This refuse contains garbage and rubbish materials like plastics, glasses, metallic cans, fibres, papers, rubbers, street sweepings, fuel residues, leaves, containers, abandoned vehicles and other discarded manufactured products.
Recent reports indicate that in United Kingdom nearly 15 million tonnes of domestic sewage are disposed-off into the land. In United States also, each sunset sees a new mountain to be precise, 4,10,000 tonnes of solid wastes. New York itself throws out 25,000 tonnes of solid stink when none of the city’s fourteen landfills, in use of more than 20 years, can take any more. Across the Atlantic, the situation of refuse is also critical.
It is estimated that in India along, about 115 million of urban population produces nearly 15 million tonnes of solid wastes causing chronic pollution of land and water, In critically polluted cities like Mumbai, Kolkata, Kanpur and Chennai, about 750 boogies are used.
Delhi, which is the third most polluted city amongst 41 critically polluted cities, collects about 3000 tonnes of garbage from its streets every day, to be thrown into its five landfills, there by polluting the land areas.
Urban domestic wastes though disposed-off separately from the industrial wastes, can still be dangerous. This is so because they cannot be easily degraded. Over population and increasing consumption have totally changed the very complexion of domestic wastes into a complex mixture of food-remains, papers, plastic and many notorious chemicals.
Other items like paints and varnishes which we use to add colour and gloss to everyday life also add poison to be urban wastes posing soil pollution problems.
The leachates from dumping sites and disposal tanks of sewage mixed with industrial effluents and wastes are extremely harmful and toxic. Actually, the leachates that oozes out of the polluted soil, contain poisonous gases along with the partly decomposed organic material especially food remnants, vegetables, toxic hydrocarbons and pathogenic microbes, many of which can be disease causing.
Pollution concentration in urban areas and unplanned industrial progress in and around these urban areas, have to a greater extent contributed to soil pollution problems in India.
About 12 crore population of India lives in cities while its six times more population lives in villages which dump their waste products into the soil, posing terrestrial and aquatic pollution hazard. It is estimated that Rs.20 crore have been spent simply for burning, compositing and thermally decomposing the refuse of Mumbai and Kolkata only.
iii. Soil Pollution by Radioactive Pollutants:
Radioactive resulting from explosions of nuclear devices, atmospheric fall out from nuclear dust and radioactive wastes (produced by nuclear testing laboratories and industries) penetrate the soil and accumulate there creating land pollution.
Radio nuclides of radium, thorium, uranium, isotopes of potassium (K-40) and carbon (C – 14) are very common in soil, rock, water and air. Explosion of Hydrogen weapons and cosmic radiations induce neutron-proton reactions by which nitrogen (N-15) produces C-14. This CI 4 participates in the carbon metabolism of plants which is then introduced into animals and man.
Radioactive waste contain several radio nuclides such as strontium-90, iodine-129, caesium-137 and isotopes of iron which are most injurious. Sr-90 gets deposited in bones and tissues instead of Calcium.
Nuclear reactor produces waste containing Ruthenium-106, Iodine-131, Barium-140 and Lanthanium-140, Caesium-144 with Promethiem-144 along with the primary nuclides Sr-90 and Cs-137. These are also produced from nuclear fission.
Cs-137 has a half-life of 30 years while Sr-90 has half-life 28 years. Rain water carry Sr-90 and Cs-137 to be deposited on the soil where they are held firmly with the soil particles by electrostatic forces.
Soil erosion and heavy rains carry away the deposited Cs-137 and Sr-90 with the silt and clay. All these radionuclides deposited on the soil emit gamma radiations. Recently, it has been indicated that some plants such as lichen and mushroom can accumulate Cs-137 and other radio nuclides which concentrates in grazing animals.
iv. Soil Pollution by Agricultural Practices:
Modern agricultural practices pollute the soil to a large extent. Today with the advancing agro-technology, huge quantities of fertilizers, pesticides, herbicides, weedicides and soil conditioning agents are employed to increase the crop yield.
Many agricultural lands have now excessive amounts of plants and animals wastes which are posing soil pollution problems. Apart from these farm wastes, manure slurry, debris, soil erosion containing mostly inorganic chemicals are reported to cause soil pollution. USA alone produces about 18 million tonnes of agricultural wastes every year.
Some of the agents responsible for this pollution are as follows:
(a) Fertilizers:
Now a days agricultural practices rely heavily on artificial fertilizers, which generally contain one or more of the plant nutrients i.e., nitrogen, phosphorous and potassium. Critical pollution problems arise mainly from their excessive application rates. Although the fertilizers are used to fortify the soil, yet they also contaminate the soil with their impurities.
When the fertilizers are contaminated with other synthetic organic pollutants, the water present in the soil may also get polluted. Generally fertilizers are retained by the soil and crop efficiently but there are some possibilities for the nitrates to be washed out due to negligence appliances in applying fertilizers to arable lands particularly in a wet spring.
These nitrates causes several undesirable effects on the water quality of low land lakes or rivers creating numerous health hazards. Reports indicate that if phosphate and nitrate concentration exceeds one part and thirty parts per hundred million parts of water respectively, it results in eutrophication, chocking the whole stretch of aquatic ecosystem.
India utilizes 16 kg per hectare of fertilizers whereas the world average is 55 kg/ha. Recently, NCA have estimated the increased use of fertilizer from 2.8 million tonnes in 1976 to 6 million tonnes in 1984 and 9.7 million tonnes in 1995.
Phosphatic fertilizer consumption rose from 2.5 mt. in 1985 to 3.4 mt in 1990. However, it is not only the increasing utilization of fertilizers but also escalated production which creates soil pollution hazards.
(b) Pesticides:
The need of increased food production due to growing population density was emphasized since long, which consequently led to manipulations of land resources. Different kinds of pesticides used to control pests are causing a stress in the natural environment.
However scattered information’s all over India on paddy fields alone have indicated that there was 108% increase in the yield of TR-8′ and 195% in ‘TN-I’ varieties when grown under plant protection umbrella using some biocides.
But from 1950 onwards the production of numerous synthesized organic pesticides have completely changed the basis and strategy of pest control. With the increasing use of pesticides as part of the newly developing agro-technology it was realized that a single pesticide did not completely eliminate all the species of target pest.
As a result, the number of commercial pesticides has increased during the past 20 years and now their number might be over 1000, which includes herbicides, fungicides, insecticides, rodenticides, nematicides, molluscicides and pesticide.
Among pesticides the most important are the chlorinated hydrocarbons e.g., D.D.T., B.H.C aldrin, endrin, dieldrin, ethion, lenthion, trithion dursban, dimethoae phosdrin and metasystox etc. The remanants of these pesticides may get absorbed by soil particles which may contaminate root crops grown in soils.
Unfortunately these pesticide residues coexist within biological system with other forms of life. The elimination of pests in the soil must inevitably produce change and disrupt the balanced natural cycles and food chains within natural ecosystems.
Residual herbicides which are applied to the soil at the time of seeding remain active for several weeks and prevent the growth of weeds in competition with the emerging germinating crop.
(c) Soil Conditioners:
Fumigants and Other Chemical Agents – In addition to the fertilizers, pesticides and biocides, soil conditioners and fumigants are also employed to the land system to increase and protect the soil fertility as well as to kill the hazardous insects. These chemical agents are reported to cause alterations in both agricultural soil areas.
They contain several toxic metals like lead, arsenic, cadmium, mercury and cobalt etc. which when applied to a land will accumulate on the soil permanently there by introducing these chemical components into growing crops.
However, researches are being carried out to synthesize pesticides of short lived degradable residues so that the persistence of the pesticide residues and their degraded product on soil, food and large crops may be reduced considerably.
(d) Farm Wastes:
Increasing population of cows, catties, pigs and poultries have resulted in considerable soil pollution. Buildings in which grazing animals are housed can be cleaned using water but the manure is also washed out and pollutes it. When these farm wastes are dumped into heaps, they may become a good breeding ground for insects and several nuisances may arise.
It has been reported that a cow produces as much organic waste as twenty people. While a pig as much as three people. Their faecal matter mainly consists of phosphates which in conjunction with nitrates cause numerous undesirable effects in the soil texture. Animal wastes contain several pathogenic bacteria and viruses which enter into plant metabolism and ultimate to man.
Even the cow dung burning is hazardous to health due to the presence of benzo-pyrene in smoke which is a cancer promoting chemical. By burning cow dung-nitrogen rich manure, which is so essential to our cultivation, is reduced to ashes.
This raw sewage has a high value of B.O.D. (200-ppm), C.O.D. (400 ppm) and nitrogen (40 ppm) while the feedlot runoff have 1000 ppm of B.O.D. 8000 ppm of C.O.D. and 700 ppm, of nitrogen.
So the animal wastes are difficult to treat and the methods applied to treat municipal wastes cannot be used for farm wastes. However, a few biological degradation methods are reported to be satisfactory in their control. Simpler methods have to be researched in future to eliminate the difficulties encountered in biological methods.
v. Soil Pollution by Chemical and Metallic Pollutants:
Number of industries including textiles, pesticides, paints, dyes, soap and synthetic detergents, tanneries, drug, batteries, cement, asbestos, rubber, petroleum, paper and pulp, sugar, steel, glass, electroplating and metal industries pour their hazardous effluents in soil and water creating disastrous effects on living organisms.
Some of the major industries and their pollutants in water:
(i) Synthetic chemical and fertilizers are a source of trace metals which are added to the soil either deliberately or as impurity. For example, As, Pb, and Cd are the common trace metals in rock phosphate, also occur in super phosphate fertilizers.
Toxic selenium species get less readily oxidised so it cannot be removed easily from soil by weathering. The selenite ion in iron rich soils forms insoluble basic ferric selenite or sorbs strongly on the iron oxides. In acidic soils of Hawaii, selenium content occurs as 20 ppm. But a very little is available to plants; while the rest Se is often found associated with sulphur.
(ii) Today various tract elements such as Fe, Co, Ni, Cu, Zn, Ba, Pb, V, Mn, Ni, As, Hg, Mo and silicon are being added to the soil in one or the other form. Mn and Fe oxides have a tendency to concentrate trace metals by isomorphous replacement of ions.
(iii) In many soils 50 to 100% of soil carbon is found complexes with clay containing organic and inorganic components which affect the soil texture, its fertility and stabilization of soil organic matter.
(iv) Presence of high levels of Na, Mg and K causes calcium deficiency in soil. Magnesium deficiency in soil have attributed to high concentration of Ca, Na and K which are added as artificial fertilizers,
(v) Excess of sulphur in soil may be absorbed in and may be involved in photosynthesis but if present at high levels pose lethal effects on crop production.
(vi) Metallic contaminates in soil are considered to be highly dangerous since they affect the production of atmospheric oxygen as well as living beings.
vi. Soil Pollution by Biological Agents:
Soil gets large quantities of human, animals and birds excreta which constitute the major source of land pollution by biological agents. Digested sewage sludge as well as heavy application of manures to soils without periodic leaching could cause chronic salt hazard to plants within a few years.
In addition to these excreta faulty sanitation, municipal garbage, waste water and wrong methods of agricultural practices also induce heavy soil pollution. Sludge’s do have faults as they contain enough live viruses and viable intestinal worms. In developing western countries, intestinal parasites constitute the most serious soil pollution problems.
The pathogenic organisms that pollute the soil may be classified into three categories as follows:
(i) Pathogenic Organisms Occurring Naturally in Contaminated Soil:
Soil has its own distinctive flora and fauna i.e. it is inhibited by bacteria, fungi, algae, protozoans, actinomycetes, nematodes, rotifers, earthworms, fungi, molluscs and arthropods etc. These organisms are important agents in increasing or decreasing the soil fertility, in altering the physical texture of the soil and attacking roots of plants.
(ii) Pathogenic Organisms Excreted by Man:
Human excreta includes pathogens such a enteric bacteria and parasitic worms. These organisms are transmitted to the man by the consumption of vegetables or fruits which are grown in the contaminated soil or by direct contact with the contaminated soil.
Beside this, insanitary habits of a large number of people have resulted in the repetition of the cycle of infection with soil transmitted pathogens from man to soil and from soil to man.
(iii) Pathogenic Organism Excreted by Animals:
This category includes pathogenic bacteria and parasitic worms excreted by animals. The animals like earthworms, millipedes, isopodes, dipterous larvae, sloug, snails including higher animals carry fungal and bacterial spores.
The disease producing organisms are transmitted from animals to soil and then from soil to man. Thus biological agents are highly responsible for heavy of soils and crops by pathogens. Now numerous methods have been developed to control pathogens. However, specific treatments are necessary for the effective removal of pathogens sewage effluents required for irrigation purposes.
Soil Pollution by Soluble Salts:
Salt accumulation has been a perpetual problem of civilization in arid and semiarid regions. Today a number of industries discharge their particular pollutants in the form of calcium sulphate, calcium carbonate, FAO states that half of the irrigated farms in the world are damaged by soluble salts deposited in soil.
Even the scientists in their attempts to determine water effluents (less water per unit of crop yield) have sometimes increased salt problems when leaching is too little. All natural water systems contain dissolved mineral substances commonly referred as soluble salts. Some rain waters, far from coastal salt sprays, may be very low in the salt content. As water flow over and through soils, it picks up salt loads.
If water rapidly evaporates as it flows on the surfaces, it results in increasing the concentration of salt. It actually happened in Colorado River in Western United States.
The erosion of salts and return flow water with salts in them add to the increased load of salt. Deicer salts, salty wastes dumped in lakes, rivers or streams and sea sprays are all the chief sources of soluble salts in soil. Actually salts washed from one field ends up in ground water or river to be used by someone else, there by spreading pollution nuisance.
How to Solve Soluble Salt Problem:
Till now, no simple solution to environmental accumulation of soluble salts is known. The only uncontested respites are oceans which are already salty and a few salt basins. One such area is the originally dry Salt on Sea of California, now used to collect drainage.
Today a number of states already have some regulation on salt problems. The main worry is, if dumping is restricted, how is the leaching to be regulated and the sources of contaminating salts to be identified. More careful use of soil as a receptor of salt will be most urgent.
However, careful irrigation to avoid excess water application will allow precipitation of Ca, Mg, SiO2 and bicarbonates. Some sulphates, carbonates and silica precipitate during drying cycles. These carbonates and silica do not re-dissolve in soils. In some waters having low sodium content but high percentage of calcium and magnesium as much as 60 to 80%, the soluble salts may get precipitated, There is a catch to this seeming light at the end of dark tunnel.
Both Na and K salts cause extensive soil dispersion and are highly corrosive to metals. Chloride is also toxic to plants in high concentration. Thus periodically, even these soils will need leaching and reclamation by removal of exchangeable sodium in soil.
Most dissolved inorganic chemicals in water are observed in soil solutions. These high concentrations of salts are extremely undesirable because they reduce or hinder plant growth, enhance corrosion of metals, make drinking water unpalatable and chronically interfere in several uses of water.
Salt pollution from agricultural run-off water is largely non-point pollution, that is pollution does not always derive from one source or point but from a combination of sources. An example is cited by lateral sewage flow and salt carried from fields in irrigation in waste water.
Salt Stress in Soil:
Saline soil containing excess of soluble salts (about 0.1 %) results in poor plants growth and productivity.
Sources of salts in soil include:
(i) Marine
(ii) Rock, and
(iii) Human activities such as industrial wastes, improper agro-techniques, municipal wastes and use of saline water for irrigation under high evaporative conditions etc.
Increasing salinity of soil threatens the civilization with ever reducing areas of normal soil for utilization of the crops. A soil is generally said to be saline if the electrical conductivity (EC) of the saturated extract of soil is greater than 4m mhos/cm at 25°C.
The predominant ions contributing to salinity are sodium and chloride, although irons such as calcium, magnesium, potassium, sulphate, borate and bicarbonate are significantly high in certain regions. This salinity status can be determined by measuring the EC of the soil solution using conductivity bridge. It is estimated that more than 20 million hectares of cultivable land is saline in India.
In saline soil, because of low osmotic potential in the root medium, plants have to develop and maintain lower osmotic potential in cells for water absorption. If the rate of water is too slow for entering in plant cells, it may cause growth reduction. Moreover, intracellular water deficit may occur due to high concentration of salts getting accumulated in apoplast.
Soil Pollution by Sugar Cane Trash in Field:
Agricultural land containing sugar cane trash is the nuclei of several pathogens, bacteria, viruses and other micro-organisms. Now restrictions on its burning, to reduce the extent of air pollution, seriously concern sugarcane growers who customarily burn tonnes of cane leaves in fields before harvest.
Project Report # 6. Effects of Soil Pollution:
Sewage and industrial effluents which pollute the soil ultimately affect human health. Various types of chemicals like acids, alkalis, pesticides, insecticides, weedicides, fungicides, heavy metals etc. in the industrial discharges affect soil fertility by causing changes in physical, chemical and biological properties.
Some of the persistent toxic chemicals inhibit the non-target organisms, soil flora and fauna and reduce soil productivity. These chemicals accumulate in food chain and ultimately affect human health. Indiscriminate use of pesticides specially is a matter of concern.
Sewage sludge has many types of pathogenic bacteria, viruses and intestinal worms which may cause various types of diseases. Decomposing organic matter in soil also produces toxic vapours.
Radioactive fallout on vegetation is the source of radio-isotopes which enter the food chain in the grazing animals. Some of these radio isotopes replace essential elements in the body and cause abnormalities e.g. strontium-90 instead of calcium gets deposited in the bones and tissues. The bones become brittle and prone to fracture. Radioisotopes which attach with the clay become a source of radiations in the environment.
Soil damage and environment degradation during surface mining is inevitable as vegetation has to be removed and huge quantities of top soil and waste rocks are to be shifted to a new location. Mining leads to loss to grazing and fertile land, soil erosion from waste dumps, sedimentation or siltation, danger to aquatic life, damage to flora and fauna as well as water and soil pollution.
A recent estimate showed that in India about 20, 000 hectares of land has been degraded from mining and another 55,000 hectares of fertile land was degraded to meet out requirement of bricks. Even open-cast coal mining also affects seriously 2,00,000 hectares of land area. It is reported that 73% of the blocks identified for exploration by CIL and Singe Reni coal-fields involve drilling in forest areas.
Mining have also resulted in displacing a large section of people from their resources base. Since the mines are mostly in forest area, they severely affect the symbiotic relationship existing between tribals and forests.
Mining activates cause ecological damage and affect natural bio-diversity leading to erosion of environmental richness. Mining would result in high evolution of carbon dioxide, enhancing greenhouse effect, acid rain, global warming and over all climatic changes.
Adopting New Techniques – Modified techniques from dig dump mining to continuous system has been adopted recently by western countries along with sequential technique. Promotion of acceptable substitutes and recycling of all metallic wastes will reduce the potential hazard and will help to achieve sustainability in the long run.
The methods are not only environmentally efficient, but cost effective also. The India Bureau of Mines, Forest Research Institute, CMPDI and universities are actively engaged to disseminate new appropriate and eco-friendly indigenous mining technologies.
Rehabilitation:
The rehabilitation strategy needs to be broad based and made interdisciplinary. Appropriate cost effective measure include: storage of top soil, selection of ecologically and socio-economically suitable species, loss of fertile land due to erosion, loss of water retentivity, improvement of hydrological regime, accelerating natural regeneration enlisting people’s participation support in afforestation, fuel wood conservation and social fencing etc.
The development plant should be blended with action under the Nation wasteland Development Programme. The plan should consider climate, rainfall pattern, soil texture, demand and supply of fodder, timber, population and other bio-mass needs.
Project Report # 7. Diseases Caused by Soil Pollution:
(i) Pathogenic soil bacteria are chronic disease carrier.
(ii) Soil proves the best medium for the growth of eggs, larvae and flies etc.
Project Report # 8. Control of Soil Pollution:
(i) Effluents should be properly treated before discharging them on the soil.
(ii) Solid wastes should be properly collected and disposed off by appropriate method.
(iii) From the wastes, recovery of useful products should be done.
(iv) Biodegradable organic waste should be used for generation of biogas.
(v) Cattle dung should be used for methane generation. Night-soil (human faeces) can also be used in the biogas plant to produce inflammable methane gas.
(vi) Microbial degradation of biodegradable substances is also one of the scientific approaches for reducing soil pollution.
(vii) Collection of waste.
(viii) Disposal of waste.
(ix) Recovery of resources: converting waste into biogas. Sanitation meeting the heat energy demand.