ADVERTISEMENTS:
After reading this article you will learn about the weathering of rocks and minerals. Also learn about the factors affecting it.
Weathering of Rocks and Minerals:
Rocks occurring within the earth remain in equilibrium with their underground environment. When the portion of the earth’s crust is upheaved (gets lifted up from beneath) or the rock/soil overlying the rocks are naturally are removed, the rock thus exposed at the surface of the earth are in a state of disequilibrium with their new environment at the surface of the earth when the rocks are acted upon by the atmospheric agents i.e. heat, cold, rain, wind and chemical action of atmospheric gases. Consequently rocks are partially/wholly disintegrated and decomposed.
All these changes which have been caused by the action of the atmospheric agents on rocks and minerals at or near the surface of the earth is called the ‘Weathering’ of rocks which may be mechanical /physical, chemical and biological. The weathering process may be mechanical or physical and chemical and biological.
ADVERTISEMENTS:
Mechanical or physical weathering of rocks breaks the rocks down to smaller and smaller pieces without appreciably altering their chemical composition. But a definite chemical change takes place by the chemical weathering of rocks. Easily decomposable minerals are decomposed; the products of the decomposition may be washed away or may recombine with each other to form new minerals.
Mechanical or Physical Processes of Weathering:
Rocks heat up during the day and cool down during the night. They are composed of minerals which differ in their coefficient of expansion. Different minerals present in the rocks expand and contract at different rates, on being heated and cooled.
Some expand and contract more than others. This differential expansion and contraction of minerals continues for long time. Consequently, cracks appear on the surface of the rocks. Ultimately, rocks break down to smaller and smaller pieces.
Rocks occurring inside the earth remain under tremendously high pressure of the rocks overlying them. When the overlying rocks are eroded away, or they are geologically upheaved to be exposed at the earth’s surface, they are relieved of the high over burden pressure.
ADVERTISEMENTS:
Consequently they expand when the outer layer of the rock may separate from the rest of the rock masses. This process is called exfoliation. In cold weather, water freezes in the cracks of rocks to form ice when tremendous pressure (about 150 tons per square foot) is exerted upon the rock masses by the expanding ice.
The cracks widen. Ice melts during the warm season and water again freezes to form ice during the next cold season, which again expands. So the width of the crack increases more due to the pressure of the expanding ice. Repetition of this process causes the rocks to gradually break down to smaller and smaller pieces.
Rocks and minerals were disintegrated by the abrasive action of glaciers as they moved under their own weight. Water, while it flowed over the rocks, dislodged smaller pieces of rocks and carried them away. Water loaded with such materials has tremendous cutting power. A rock rolls in running water and becomes rounded. Wind, when it carries coarser particles, scrapes rocks and carries away the finer particles.
Chemical Process of Weathering:
An atmospheric gas like carbon dioxide, sulphur dioxide, nitrogen peroxide water vapour, and oxygen react with the minerals contained in the rocks at different temperatures, and changes them to new minerals by the following processes:
ADVERTISEMENTS:
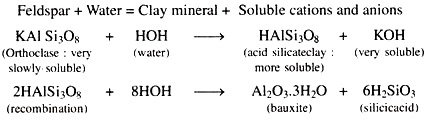
1. Hydrolysis:
The reaction of the minerals with water is called hydrolysis of minerals as shown in the following equation.
Bauxite and Silicic acid may combine with each other to form clay minerals like Kaolinite.
ADVERTISEMENTS:
2. Hydration:
Hydrogen and hydroxyl ions are rigidly attached with the crystals of the minerals to become an integral part of them. This phenomenon is called hydration of minerals. For example, red haematite reacts with water to form yellow limonite
When micas become hydrated some hydrogen and hydroxyl ions move in between plate-like units of mica expand it and make it more porous.
ADVERTISEMENTS:
3. Carbonation:
The process by which carbonic acid reacts with minerals and changes them to new minerals, as shown below is called carbonation:
4. Solution:
Rain water gradually dissolves sparingly soluble minerals as shown above. Heavy rain washes the calcium and the bicarbonate ions down, the above reversible reaction proceeds in the forward direction. So the sparingly soluble calcium carbonate is gradually dissolved in water.
5. Oxidation:
The process by which electrons are lost from ions whose positive charge is increased is called oxidation. For example, ferrous iron loses an electron to become ferric iron
A compound is said to be oxidized when it combines with oxygen as shown in the following equation:
Ferromagnesian minerals contain ferrous ions which are easily oxidized to the ferric ion. So some positive ions must go out of the crystal lattice of the mineral in order to maintain its electrical neutrality.
So some position in the crystal lattice of the mineral becomes empty, facilitating the entry of ions into it and exist of some ions from it.
Ferromagnesian minerals are rapidly decomposed this way:
Biological Process of Weathering:
Some lower forms of plants like mosses, algae and lichens (Sec. 8.4) grow even on bare rocks and respire producing carbon dioxide, which combines with water to form the carbonic acid that decomposes minerals contained in the rock to form the clay. These organisms are short lived. They die and their bodies quickly decay to add organic matter to rocks, on which another crop of microorganisms grow.
Higher plants also grow within the crevices of rocks, widened by the pressure exerted by the growing roots on rock masses. They also add organic matter to the rocks which decomposed to form organic acids decomposed rock minerals to form more clay.
Big masses or rocks are also disintegrated by human activities. The physical process of weathering of soil mineral is accelerated by tillage operations. Application of lime and fertilizers to soils hastens the decomposition of the soil minerals.
Animals e.g. moles and rats make burrows when the soil minerals become smaller by abrasion. Earthworms eat large amounts of organic matter along with fine clay. The earthworm casts are ideal environments for quicker decomposition of the solid minerals.
Factors Affecting Weathering of Rocks and Minerals:
(i) Climate:
The physical or mechanical process of weathering dominates over the chemical process under conditions of low rain fall of when the sizes of the minerals particles are decreased without much change in their chemical composition. But the minerals are decomposed by high rainfall and high rainfall conditions in humid regions.
(ii) Physical Characteristics:
Rocks composed of large crystals are easily disintegrated when the temperature frequently change. Hence physical or mechanical weathering dominates. But when the rocks are composed of small crystals of minerals, more surface area is available for a chemical reaction. Hence the chemical process of weathering dominates over the mechanical process of weathering.
(iii)Chemical Composition:
Dark coloured ferromagnesian minerals containing ferrous ions are easily decomposed, because ferrous ions are oxidized to ferric ions. So some ions must leave the crystal structure of the mineral to balance it electrically thus leaving empty spaces in the crystal which reduce the electrostatic forces that binds the different parts of the crystal together. They also become more susceptible to acid attack and dissolution. The crystal structure is gradually weakened and ultimately broken down.