ADVERTISEMENTS:
On the basis of number and arrangement of tetrahedral and octahedral sheets, silicate clays are classified into 3-different groups: 1. 1:1 Type Clay Minerals 2. 2:1 Type Minerals 3. 2: 1:1 Type Minerals.
1. 1:1 Type Clay Minerals:
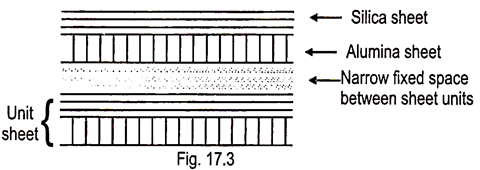
Such silicate clay is made up of one silica sheet and one alumina sheet combined. In soils, Kaolinite is the prominent member of 1:1 type group. Others are Halloysite, Nacrite & Dickite.
The two sheets are held together by oxygen anions (O2-) mutually shared by Si4+ and Al3+ in their respective sheets. These units are in turn held together rigidly by H- bonding.
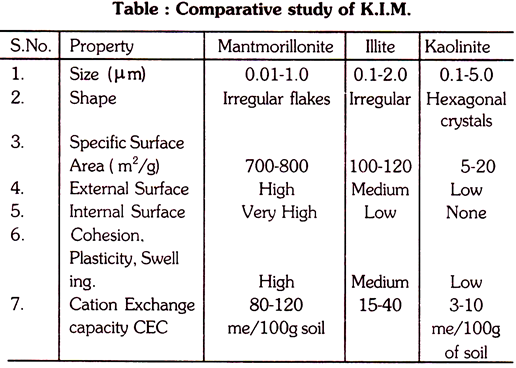
Thus lattice is fixed due to strong bonding and no expansion between two units when wetted; cations and water do not enter between the units; little isomorphic substitution (CEC); plasticity, cohesion, shrinkage and swelling are low. Kaolinite does not exhibit colloidal properties of a high order of intensity. Size of kaolinite units ranges from 0.1-50.μm in width (but majority has 0.2-2.0. μm); Pseudohexagonal in shape. Kaolinite is bigger than other group.
Halloysite has sheets of water between these layers and tubular crystals thus plasticity, shrinking and swelling exceed slightly than of Kaolinite. Total surface area per unit mass is only 15 m2/g.
2. 2: 1 Type Minerals:
Octahedral sheet (alumina) is sandwiched between two tetrahedral (silica) sheet.
(i) Expanding Minerals:
ADVERTISEMENTS:
It includes Smectite and Vermiculite groups.
Smectite Groups:
These include montmorillonite, Beidellite, Nontrornite and saponite. These are noted for interlayer expansion on wetting due to entering of water causing swelling. Among these, Montmorillonite is prominent in soils. Smectte is composed of 2:1 type layers and these layers are loosely held together by very week Oxygen to Oxygen and Cation to Oxygen linkages.
ADVERTISEMENTS:
There is little attraction between O2- in the bottom silica sheet and those in top silica sheet. Therefore exchangeable cations and associated water molecules are attracted between the layers causing expansion. Mg 2+ replaces Al3+ in some sites of alumina sheet and Al3+ replaces Si4+ in some sites of silica sheet.
These substitutions give rise to negative charges which account for high Cation Exchange Capacity (CEC). CEC is defined as the amount of a cation species bound at pH 7 (neutral pH) and is expressed as cmol (P+) kg-1. Cmol means centimol. Previously it was expressed as me/100g soil i.e. mili equivalent. These negative charges are satisfied by a swarm of cations. The specific surface or total surface area per unit mass is 700-800m2/g.
Montmorillonite:
It is most common smectite in soils where Mg2+ is substituted for Al3+ in alumina sheet (Octahedral).
ADVERTISEMENTS:
Beidellite:
Substitution of Al3+ for Si4+ in silica sheet (Tetrahedral).
Nontronite:
Fe3+ (trivalent Iron) dominates the alumina sheet (octahedral) and some Al3+ replace Si4+ in silica sheet.
ADVERTISEMENTS:
Vermiculite:
It has similar structural characteristics to smectite. Most vermiculites are dioctahedral (Al- dominated sheet) and have same isomorphic substitution to smectite in the tetrahedral sheets, considerable substitution of Al3+ for Si4+ accounts for very high negative charges.
The water molecules, Mg2+ and other cations are strongly adsorbed in the interlayer which act as bridge holding it unites together. So degree of swelling is considerably less than of smectite, and therefore called limited expansion clay mineral. Expansion is more than of kaolinite but much less than of montmorillonite (i.e. Kaolinite- Vermiculite- Montmouillonite).
The CEC of Vermiculites exceeds that of all other silicate clays due to very high negative charges. (The CEC in ascending order- Kaolinite- Illite – Montmouillonite- Vermicute- Humus). The Vermiculite crystals are larger than that of montmorillonite but much smaller than of kaolinite.
(ii) Non- Expanding Minerals:
In clay, fine grained micas (or Illite) are found, Illite has 2:1 type crystal. About 20% Si4+ of silica sheet is replaced by Al3+ which results in high net negative charge in tetrahedral (silica) sheet. To satisfy this charge K+ are strongly attracted in the interlayer space. Thus K+ acts as binding agents preventing expansion. Therefore hydration, CEC, swelling shrinking & plasticity are much less than of montmorillonite.
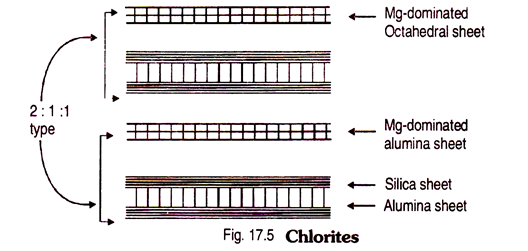
3. 2:1:1 Type (or 2:2) Type Mineral:
Example – chlorites.
Chlorites are basically ferro- magnesium silicates with some aluminium present.
Chlorites have an extra layer of Mg-dominated alumina sheet say Brucite [Mg (OH)2]. But Mg2+ also dominates the alumina (trioctahedral) sheet of 2:1 type minerals. Thus crystal unit has two silica sheets (tetrahedral) and two magnesium dominated alumina sheet. That’s why sometimes it is also called 2:2 type clay mineral. In other words chlorites are basically silicates of Mg with some iron and aluminium. The CEC is about same as of Illite, non-expanding nature.
Sources of Negative Charge on Silicate Clays:
1. Isomorphic Substitution:
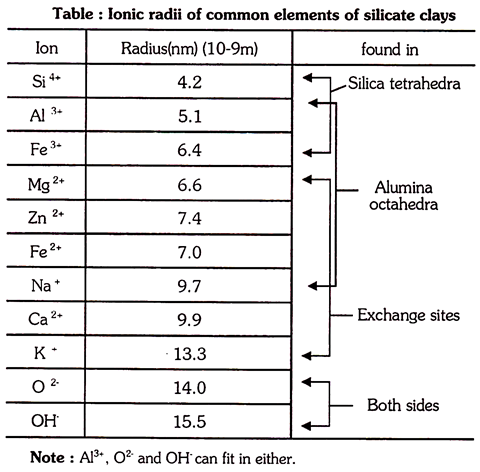
Al3+ is slightly larger than Si4+ hence Al3+ can fit into the centre of tetrahedron in the place of Si4+ without changing the basic structure of crystal.
In octahedron, Fe and Zn can fit into the position of Al or Mg. Positioning of Mg2+ in place of Al3+ or Al3+ in place of Si4+ leaves unsatisfied negative charges from Oxygen anions in the sheets which account for overall negative charges from Oxygen anions in the sheets which account for overall negative charge of clay.
Isomorphic substitution is of great significance in 2:1 type. The resultant negative charge is far in excess of that resulting from broken crystal edges of these minerals. Unlike the charge associated with the exposed crystal edges, those resulting from ionic substitution are not dependent on pH.
The isomorphous substitution of any cation having lower charge results in an increase in positive charge. It is commonly occurred in trioctahedral layers (Mg – dominated sheets) when Mg2+ is replaced by Fe3+/Al3+
Isomorphic Substitution results in Constant (Permanent) Charges.
2. Exposed Crystal Edges:
i. Ionisation of hydroxyl groups
ii. Ionisation of carboxyl and phenolic groups
O2- and OH– groups are exposed at the broken edges and flat external surface as in Kaolinite- At pH> 7, the hydrogen of these hydroxyls dissociates slightly and the colloidal surface is left with a negative charge carried by oxygen.
The loosely held H+ is readily exchangeable hence called pH-dependent charge of inorganic colloids. This phenomenon apparently accounts for most of the CEC of 1:1 type colloidal clays and for organic colloids.
Ionisation of carboxyl (-COOH) or phenolic (C6H5OH) groups is the chief source of negative charges on humus micelles. With the increase in pH, extent of negative charge is increased, therefore is called pH-dependent negative charge or variable charge. As the soil pH increases, more OH– ions are available to force the reactions to the right; and negative charge increases.
Second possible point is that at high pH, complex aluminium hydroxy ions e.g. Al(OH)2+ is removed because these ions react with OH– to form insoluble Al(OH)3, thereby releasing negatively charged sites. But at low pH it blocks the negative sites and make them unavailable for cation exchange (the process of exchange of cations between solid and liquid phases).
In the soils of temperate climates where 2:1 type clays are common, the permanent negative charges are usually dominant. In highly weathered soils of tropics where 1:1 type silicate clays, Iron and Aluminium oxides dominate and in soils high in O.M. the variable negative charges are more common.





Comments are closed.