ADVERTISEMENTS:
After reading this article you will learn about the chemistry of hydrogen and aluminium in the development of soil acidity.
It is evident that hydrogen and aluminium both contributes soil acidity. Hydrogen ions contribute soil acidity directly while aluminium ions do so indirectly through hydrolysis. Therefore, it is essential to know the chemistry of aluminium for the development of soil acidity.
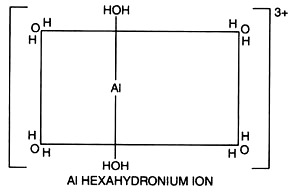
In aqueous solutions Al3+ does not remain as a free ion, but it is surrounded by six molecules of water forming hexaquoaluminium compound [Al(H2O)63+].
As the soil water solution becomes less acid (has more OH–), one or more aluminium held water molecules ionizes H+ ions, which are less attracted to the oxygen of water molecules held to the aluminium; the ionization leaves a hydroxyl (OH–) ion attached to the aluminium, which neutralizes some of its charge.
The aluminium ion becomes successively less positively charged by such ionization. At different pH levels, these are the forms of aluminium in soil.
Solution:
So total aluminium in soil solution is pH dependent and must include above hydrolysis species as well. These aluminium hydrolysis products can be re-adsorbed by the clay minerals causing further hydrolysis with the release of hydrogen (H+) ions in the soil solution and thereby develops soil acidity (lower soil pH). The relationship between pH and aluminium species is depicted in Fig. 14.4.
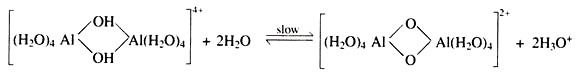
The monomelichexaquoaluminium [Al(H2O)63+] ion is exchangeable. However, because of its trivalent charge it will be retained strongly by soil colloids. The hydroxyaluminium ions tend to polymerize rapidly to form large multi-charged units.
Such polymerization is favoured in the presence of soil colloid surfaces.
The mechanism for their formation is the sharing of hydroxyl (OH”) groups by adjacent aluminium ions as shown in the following equations:
On aging, they release either hydrogen (H+) ions or polymerize further and become tightly bound to colloids surfaces. These multi-charged polymers are positively charged and are essentially non-exchangeable. Cation exchange capacities of the soil colloids can be affected by the formation of these positively charged polymers on their surfaces.
At high pH values the polymeric positive charges become less and can even become negative as a result of the dissociation of hydrogen (H+) ions or protons.
As a result, cation exchange capacities of the soil colloid polymer complex will increase. Decreasing pH in soils with large amounts of these aluminium polymers closely associated with soil colloids will lower cation exchange capacities by increasing the positive charge on the polymers.
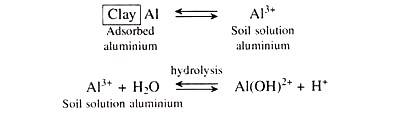
Under strongly acid soils, the adsorbed aluminium is in equilibrium with aluminium (Al3+) ions in the soil solution and that aluminium ions in solution produces hydrogen (H+) ions through the process of hydrolysis as follows:
Under moderately acid soils, the percentage of base saturation and pH values are somewhat higher. At such higher pH values, aluminium exists as aluminium hydroxy ions and again on hydrolysis liberates hydrogen (H+) ions in the soil solution.
Al(OH)2+ + H2ODAl(OH)2+ + H+
Al(OH)2++ H2ODAl(OH)30+H+