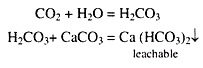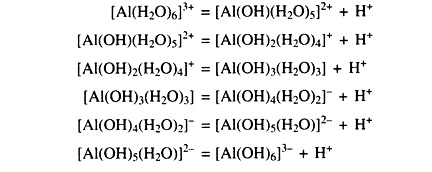ADVERTISEMENTS:
After reading this article you will learn about:- 1. Definition of Soil Acidity 2. Source of Soil Acidity 3. Kinds 4. Problems 5. Amelioration.
Definition of Soil Acidity:
An acid is a substance that tends to give up protons (hydrogen ions) to some other substance. Conversely, a base in any substance that tends to accept protons (hydrogen ions). Soil acidity may be defined as the soil system’s proton (H+ ions) donating capacity during its transition from a given state to a reference state.
Soil acidity involves intensity and quantity aspects. The intensity aspect is universally characterised by the measurements of H+ ion activity, expressed as pH. The quantity aspect is characterised, directly or indirectly, by the quantity of alkali required to titrate soil to some arbitrarily established endpoint. Soil acidity is a major problem in relation to plant growth and therefore, acid soils are called a problem soil.
Source of Soil Acidity:
ADVERTISEMENTS:
Acid soil is a base unsaturated soil which has got enough of adsorbed exchangeable H+ ions so that to give soil a pH of lower than 7.0. The following important sources which are responsible for the development of acidic soils.
(i) Leaching due to Heavy Rainfall:
Generally acid soils are common in all regions where rainfall or precipitation is high enough to leach appreciable amounts of exchangeable bases from the surface soils and relatively insoluble compounds of Al and Fe remains in soil. The nature of these compounds are acidic and its oxides and hydroxides react with water (H2O) and release hydrogen (H+) ions in soil solution and soil becomes acidic.
Besides, when the soluble bases are lost, the H+ ions of the carbonic acid and other acids developed in the soil replace the basic cations of the colloidal complex. As the soil gets gradually depletes of its exchangeable bases through constant leaching, it gets de-saturated and becomes increasingly acid.
(ii) Acidic Parent Material:
Some soils have developed from parent materials which are acid, such as granite and that may contribute to some extent soil acidity.
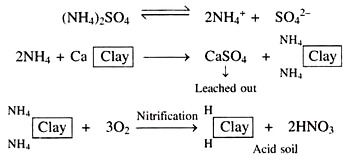
(iii) Acid Forming Fertilizers and Soluble Salts:
The use of ammonium sulphate (NH4)2SO4 and ammonium nitrate, NH4NO3 increases soil acidity. Ammonium (NH4) ions from (NH4)2SO4 when applied to the soil replace calcium (Ca2+) ions from the exchange complex and the calcium sulphate (CaSO4) is formed and finally leached out.
Besides, basic portion of ammonium sulphate, (NH4)2SO4 is NH4 and it undergoes biological transformation in the soil and form acid forming nitrate (NO3–) ions. Similarly, sulphur also produce acid forming sulphate (SO42-) ions through oxidation.
Divalent cations of soluble salts usually have a greater effect on lowering soil pH than monovalent metal cations.
(iv) Humus and Other Organic Acids:
Humus materials in soils occur as a result of microbiological decomposition of organic matter and contain different functional groups like carboxylic (-COOH), phenolic (-OH) etc. which are capable of attracting and dissociating hydrogen (H+) ions.
ADVERTISEMENTS:
During organic matter decomposition, humus, organic acids and different acid salts may also be produced and also concentration of CO2 increased. The increased concentration of CO2, hydrolysis of acid salts and various organic acids combinedly increased the total acidity of soil.
(v) Aluminosilicate Minerals:
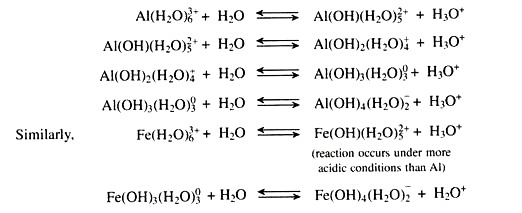
At low pH values most of the aluminium (Al) is present as the hydrated aluminium ions (Al3+) which undergoes hydrolysis and release hydrogen (H+) ions in the soil solution.
It is also possible that structural OH– ions at corners and edges may dissociate hydrogen (H+) ions and develop soil acidity.
ADVERTISEMENTS:
(vi) Carbon Dioxide (CO2):
Soil containing high concentration of CO2, the pH value of such soil will be low i.e. the soil becomes acidic. Root activity and metabolism may also serve as sources of CO2 which ultimately helps the soil to become acidic.
(vii) Hydrous Oxides:
These are mainly oxides of iron and aluminium. Under favourable conditions they undergo stepwise hydrolysis with the release of hydrogen (H+) ions in the soil solution and develop soil acidity.
(viii) Aluminium and Iron Polymers:
The Al3+ ions displaced from clay minerals by cations are hydrolyzed to monomelic and polymeric hydroxy aluminium complexes. Hydrolysis of the monomelichexaquoaluminium or iron forms are illustrated by the following stepwise reactions with the liberation of hydronium ion (H3O+) and lower soil pH. Each successive step occurs at a higher pH.
Kinds of Soil Acidity:
Broadly soil acidity may be of two kind’s viz:
(i) Active acidity and
(ii) Potential reserve/ exchange acidity.
The nature of soil can be illustrated as follows:
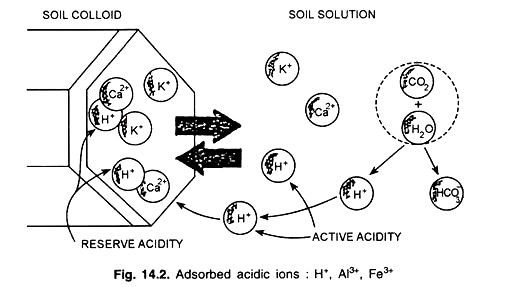
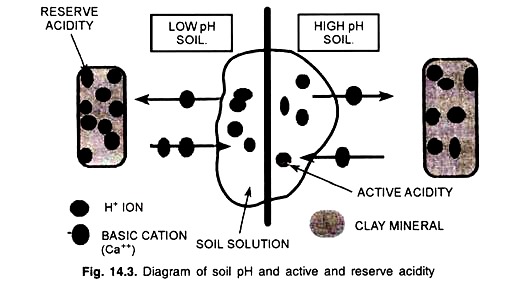
(i) Active Acidity:
Active acidity may be defined as the acidity develops due to hydrogen (H+) and aluminium (Al)3+ ions concentration of the soil solution. The magnitude of this acidity is limited.
(ii) Exchange Acidity:
Exchange acidity may be defined as the acidity develops due to adsorbed hydrogen (H+) and aluminium (Al3+) ions on the soil colloids. The magnitude of this exchange acidity is very high. However, residual acidity may be included to the total acidity.
Residual acidity may be defined as the acidity which remains in the soil after active and exchange acidity has been neutralized. It is associated with aluminium-hydroxy ions and with H and Al atoms that are bound in non-exchangeable forms by organic matter and silicate clay.
An equilibrium relationship between exchange and active acidity on 2: 1 type colloid is depicted in Fig. 14.1
Total Acidity:
The total acidity is summation of active, exchange and residual acidity. It can be written as,
Total acidity = Active acidity + Exchange acidity + Residual acidity.
Therefore, total soil acidity depends on the active, exchange and residual acidity of the soil.
Active acidity – Soluble acidity, in the solution.
Reserve acidity – Adsorbed acidity, on the surface of particles.
Why are the trivalent ions acidic?
Problems of Soil Acidity:
Problems of soil acidity may be divided into three groups which are as follows:
1. Toxic effects
(a) Acid toxicity
(b) Toxicity of different nutrient elements
2. Nutrient availability
(a) Non-specific effects
(b) Specific effects
(i) Exchangeable bases
(ii) Nutrient imbalances
3. Microbial activity.
1. Toxic Effects:
a. Acid Toxicity:
The higher hydrogen ion concentration is toxic to plants under strong acid conditions of soil. The acid toxicity includes possible toxicities of acid anions as well as H+ ions.
b. Toxicity of Different Nutrient Elements:
Iron and Manganese:
The concentration of these two ions (Fe2+ and Mn2+) in soil solution depends upon the soil reaction or pH, organic matter and intensity of soil reduction. Due to increase in organic matter content in the soil the population of soil microbes increases and very rapidly used up the soil oxygen and results reduction of soil.
As a result of soil reduction, the nutrient elements like Mn4+ and Fe3+ reduce to Mn2+ (manganous manganese) and Fe2+ (ferrous iron) respectively and increases their concentration to a very high and toxicity of those elements develops. Due to such toxic effects, a physiological disease of rice is found in submerged soils which are popularly known as browning disease.
Toxicity of Aluminium (Al):
The toxicity of aluminium may be greatly influenced by the accompanying cations. The toxicity of aluminium tends to decrease with an increase in the concentration of other cations such as calcium. Aluminium toxicity is a problem in both upland and lowland soils.
Aluminium toxicity in soils affects plant growth in various ways:
(i) It restricts the root growth.
(ii) It affects various plant physiological processes like division of cells, formation of DNA and respiration etc.
(iii) It restricts the absorption and translocation of some important nutrient elements from soil to the plant like phosphorus, calcium, iron, manganese etc.
(iv) It causes wilting of plants,
(v) It also inhibits the microbial activity in the soil.
2. Nutrient Availability:
a. Non-specific effects:
It is associated with the inhibition effect of root growth and thereby affects the nutrient availability.
b. Specific Effects:
i. Exchangeable Bases:
There are two aspects of availability of exchangeable bases i.e. ion uptake process and the release of bases from the exchangeable form may be adversely affected due to soil acidity. Due to complementary ion effect exchangeable bases are released preferentially in a fractional exchange. Deficiency of bases like Ca2+ and Mg2+ are found in acid soils.
ii. Nutrient Imbalances:
It is evident that soluble iron, aluminium and manganese are usually present in their higher concentrations under moderate to strong acid soils. Phosphorus reacts with these ions and produces insoluble phosphatic compounds rendering phosphorus unavailable to plants. Besides these, fixation of phosphorus by hydrous oxides of iron and aluminium or by adsorption, the availability of phosphorus is decreased.
In acid soils, iron, manganese, copper and zinc are abundant, but molybdenum is very limited and unavailable to plants. In acid soils having very low pH, the availability of boron may also be decreased due to adsorption on sesquioxides, iron and aluminium hydroxy compounds. Nitrogen, potassium and sulphur become less available in an acid soil having pH less than 5.5.
3. Microbial Activity:
It is well-known that soil organisms are influenced by fluctuations in the soil reaction. Bacteria and actinomycetes function better in soils having moderate to high pH values. They cannot show their activity when the soil pH drops below 5.5. Nitrogen fixation in acid soils is greatly affected by lowering the activity of Azotobacter sp.
Besides these, soil acidity also inhibits the symbiotic nitrogen fixation by affecting the activity of Rhizobium sp. Fungi can grow well under very acid soils and caused various diseases like root rot of tobacco, blights of potato etc.
Amelioration of Soil Acidity:
In general the fertility status of acid soils is very poor and under strongly to moderately acidic soils the plant growth and development affect to a great extent. The crops grown on such problematic soils do not give remunerative return rather it lowers down the yield to a great extent.
Because of the limited land resource it needs judicious management practices so that yield of different crops can be increased. So one of the most important and practically feasible management practices is the use of lime and liming materials to ameliorate the soil acidity.
The addition of lime raises the soil pH to some prescribed value. This value is usually in the range of pH to some prescribed value. This value is usually in the range of pH 6.0 to 7.0, since this is an easily attainable value within the optimum range of most crop plants.
Lime requirement of an acid soil may be defined as the amount of liming material that must be added to raise the pH to some prescribed value. This value is usually in the range of pH 6.0 to 7.0, since this is an easily attainable value within the optimum range of most crop plants.
Liming Reactions:
Lime reactions in soils depend upon the nature and the fineness of the liming materials. Lime is usually applied to soils in the form of ground limestone. Limestone’s can be classified as calcitic (CaCO3), dolomite [CaMg(CO3)2] or a mixture of the two.
Both of these limestone’s are sparingly soluble in pure water but do become soluble in water containing carbon dioxide. The greater the partial pressure of carbon dioxide in the system, the more soluble the limestone becomes. Dolomite is somewhat less soluble than calcite.
The reaction of limestone (CaCO3) can be written as:
In this way hydrogen ions (H+) in the soil solution react to form weakly dissociated water, and the calcium (Ca2+) ion from limestone is left to undergo cation exchange reactions. The acidity of the soil is, therefore, neutralized and the per cent base saturation of the colloidal material is increased.
The process of changing pH by the addition of lime [Ca(OH)2] is illustrated in Fig. 14.7.
Factors Affecting Liming Reactions:
Various environmental factors affect the rate of limestone reaction.
A few of the important factors are being discussed here:
(i) Moisture:
The greater the amount of moisture, the more rapid is the rate of reaction. Obviously moisture must be present before the solubility reaction can occur. As moisture increases, the degree of aeration are reduced resulting an increase in the concentration of carbon dioxide (CO2) in the soil air and thereby increases the rate of reaction. The increased moisture would also allow for a greater volume of solution and, therefore, a lower concentration of reaction end products. Since the reaction is an equilibrium reaction, the accumulation of end products would reduce reaction rate over time.
(ii) Temperature:
Lime and liming materials react more rapidly at high that at low temperatures. This effect is probably related to diffusion rates of end products away from the reaction sites.
(iii) Amount of exchange acidity:
The amount of exchange acidity present in the soil affects reaction rate. If a soil has a high lime requirement and if a sufficient quantity of limestone is added to neutralize the acidity present, the initial reaction will be quite rapid. However, as the acidity becomes neutralized, the rate of reaction decreases and finally, as neutrality is approached becomes almost negligible.
Liming Materials:
Liming materials may be defined as materials that are necessary for the neutralization of soil solution hydrogen (H+) ions. The materials commonly used for the liming of soils are the oxides, hydroxides, carbonates and silicates of calcium or calcium and magnesium.
The presence of only these elements does not consider a material as a liming compound. In addition to these compounds, the accompanying anion must be one that will reduce the activity of hydrogen (H+) ions and hence aluminium in the soil solution. These are called “Agricultural liming materials”.
Why Gypsum is not considered as a Liming Material?
Gypsum is not considered as liming materials because on its application to an acid soil it dissociates into calcium (Ca2+) and sulphate (SO42-) ions:
CaSO4DCa2+ + SO42-
The accompanying anion is sulphate and it reacts with soil moisture produces mineral acid (H2SO4) which also increases soil acidity instead of reducing soil acidity.
Besides this, calcium (Ca2+) in the gypsum after dissociation will result in replacement of adsorbed aluminium (Al3+) in a localized soil zone (when gypsum applied as band placement) with a significant lowering of soil pH. Therefore, gypsum does not qualify as a liming material.
Kinds of Liming Materials:
There are various kinds of liming materials that are used for the correction of soil acidity.
(i) Oxides of lime:
It is normally called burned lime or quick lime. Oxide of lime is more caustic than limestones.
Burned lime is produced by heating limestone and dolomite as follows:
(ii) Hydroxides of lime:
It can be produced by adding water to burned lime and is called slaked lime.
It is more caustic than burned lime (CaO).
If it is kept open in the moist air, then combination of calcium hydroxide occurs as follows:
Ca(OH)2 + Co2→ CaCO3+ H2O
In case of Mg(OH)2
Mg(OH)2 + CO2→ MgCO3 + H2O
(iii) Carbonates of lime:
These are by products of certain industries and so the content of calcium and magnesium varies. The two important minerals are found in this group- Calcite (CaCO3) and dolomite [CaMg(CO3)2].
(iv) Slags:
These are generally three types of slags that are found important:
(a) Blast furnace slag:
It is a by-product of the manufacture of pig iron. As a liming material, this slag behaves essentially as calcium silicate. The neutralizing value of blast furnace slags ranges from about 75-90%.
(b) Basic slag:
It is a by-product of the basic open-hearth method of making steel from pig iron, which in turn is produced from high phosphorus iron ores. The impurities in the iron, including silica and phosphorus are fluxed with lime and the basic slags are produced. Its neutralizing value ranges from 60-70%.
(c) Electric furnace slag:
This is produced from the electric furnace reduction of phosphate rock during preparation of elemental phosphorus. This product is largely calcium silicate and is used as a liming material.
(v) Other liming materials:
Coral shell, chalk, wood ash, press mud, by-product material of paper mills, sugar factories, fly ash and sludge etc. are considered as liming materials and also used for the amelioration of soil acidity.
Efficiency of Liming Materials:
The efficiency of liming materials can be judged on the basis of following important factors because of varying neutralizing capacity of different liming materials:
(i) Neutralizing value (N.V.) or calcium carbonate equivalent of liming materials,
(ii) Purity of liming materials and
(iii) Degree of fineness of liming materials.
(i) Neutralizing value (N.V.) or Calcium Carbonate Equivalent (CCE):
Calcium carbonate equivalent (CCE) is defined as the acid—neutralizing capacity of an agricultural liming material expressed as a weight percentage of calcium carbonate.
Example:
Calculate the CCE of dolomite, CaMg(CO3)2.
Solution:
In dolomite, there is mixture of CaCO3 and MgCO3. Both are almost equally effective in neutralizing soil acidity. Considering the materials as two separate liming materials present in the dolomite. So the molecular weight of dolomite may be considered as 184/2 = 92 since 84 gms of MgCO3 will neutralize the same amount of acid as 100 gms of CaCO3.
So,
= 100/92 × 100 = 108.7
Neutralizing value or CCE of some liming materials is given in table 14.3.
(ii) Purity of liming materials:
The more purer the liming material, the higher will be its effectiveness for the amelioration of soil acidity.
(iii) Degree of fineness of liming materials:
The rate of reaction of liming materials with an acid soil depends upon its fineness because finer materials increase the surface contact with the soil. If the liming materials are coarse, the reaction will be slight. The magnitude of such reaction as affected by the fineness of liming materials can be evaluated by measuring the change of soil pH. The amount of finer fraction of liming materials will be required much less as compared to coarser fractions of the material to achieve a certain pH.
The fineness is measured in terms of the ability of a material to pass through a sieve having 60 holes of equal size in one linear inch. Such a sieve is called a 60 mesh sieve and a material passing through such a sieve is allotted 100% efficiency rating.
One such scale used in Ohio (USA) is given below:
Per cent Effective Calcium Carbonate (ECC) or (Neutralizing Index):
The effective calcium carbonate (ECC) rating of a limestone or liming materials is one product of its calcium carbonate equivalent (CCE) and the fineness factor. The fineness factor is the sum of the product of the percentage of material in each of the three size fractions multiplied by the appropriate effectiveness factor.
Per cent ECC or N.I. = CCE × fineness factor
Chemical Reactions between Liming Materials and Acid Soils during the Process of Amelioration:
(i) Oxides of lime (CaO):
When oxides of lime like CaO are applied to an acid soil, it reacts almost immediately as follows:
(ii) Hydroxides of lime Ca(OH)2:
When hydroxides of lime like Ca(OH)2 is applied for the reclamation of an acid soil, the following chemical reaction takes place:
(iii) Carbonates of lime (CaCO3):
When carbonates of lime like calcite is applied to an acid soil, a part of CaCO3 undergoes solution and combines with H2CO3 to form soluble Ca(HCO3)2.
This calcium bicarbonate in solution form reacts with the soil colloids with the evolution of CO2 as follows:
And the rest portion of the limestone goes in close contact with the soil colloids in solid condition as follows:
(iv) Basic slag:
Basic slag can also be used as liming material when it is applied to an acid soil the following chemical reaction takes place.
The metasilicic acid is weakly dissociated, much less so that the clay absorbed hydrogen (H+) ions and the pH of the soil is raised.
Besides these chemical amendments, some other management practices have been found to be effective for the amelioration of an acid soil namely:
(i) Use of basic fertilizers like sodium nitrate, basic slag etc.
(ii) Reducing leaching of basic cations by following appropriate soil and water management practices, and
(iii) Acid tolerant crops like rice, potato, tea wheat, sweet potato, maize, brinjal etc. should be cultivated.
Lime Requirement and Liming Factor:
Once a soil is found to be acidic and then it requires some management practices either chemical amendment with some liming materials or some cultural management practices in order to improve its fertility status through the modification of soil reaction to a favourable position.
It has been found that the correction of soil acidity as well as improvement of soil fertility through liming is most effective. The different intensity of soil acidity required different amount of lime. So determination of lime requirement from an acid soil is prerequisite in a liming programme.
The desirable soil pH range for most of the field crop is 6.0 to 7.0. The amount of lime required to be added to acidic soil to raise the pH of that soil to a desired value is known as lime requirement. Shoemaker et al. (1961) buffer method is used for the determination of lime requirement of an acid soil. (Ref. Shoemaker, H.E., -Mclean E.O. and Pratt, P.E (1961)).
Buffer method for determining lime requirement of soils appreciable amount of extracted aluminium. (Proc. Soil Sci. Soc. Amer. 25: 274). Lime requirement in terms of pure calcium carbonate can be observed from the Table 14.4.
Liming Factor:
‘Liming factor’ may be defined as the factor by which the actual amount of lime can be calculated from the estimated theoretical amount of lime. This factor varies from 1 to 3, depending on rate of limestone solution, plant uptake and leaching during the reaction period. A liming factor of 1.5 to 2.0 is generally used when the theoretical amount of lime necessary to bring the soil to a given pH is converted to the actual amount of limestone to be added in the field.
Methods of Applying Lime:
The application of small amounts of lime of soil in every year or twice in a year has been found to be most effective. But this involves the application cost of the liming material. Generally lime should be applied well ahead of the crop cultivation and broadcasted limes are to be well mixed with the whole plough layer soil so that liming reaction can occur in a faster rate.
The usual liming practice consists of a compromise between what is most effective and what is cheapest per ton of lime applied. When both surface and sub-surface soils are strongly acidic e.g. ultisols, it sometimes pays to incorporate lime to a depth of about 30 cm (12 inches). Application of lime of no till field soils may not be as effective as application to an equivalent cultivated soil.
Lime Balance Sheet:
When a soil has had its acidity corrected by lime, how often must lime be added and how much is needed to keep the soil pH suitable? The answer depends upon the rate of lime loss.
Lime is neutralized or lost from the soil by six activities:
(i) Neutralization by acid-forming fertilizers, a rapid change.
(ii) Neutralization by the acid formed by CO2 in water (from organic matter decomposition), a slow process.
(iii) Leaching a relatively slow change.
(iv) Removal in harvested or grazed crops; relatively slow loss.
(v) Erosion, a top soil is lost with its higher base saturation and often leaving more acidic subsoil to be limed.
(vi) Neutralization by acids dissolved in precipitation, resulting from oxides of sulphur fumes from manufacturing plants, a slow process.
Effect of Over Liming:
When excessively large amounts of lime applied to an acidic soil the growth of plants is affected by influencing either one or many of these following causes:
(i) Deficiency of iron, copper and zinc will occur.
(ii) Phosphorus and potassium availability will be reduced.
(iii) Due to high OH– ion concentration by over liming, root development will be inhibited in association with tip swelling brought about by hydrations. Due to dehydrating properties of boron, it acts as a protective agent for excess OH– ion concentration.
(iv) Due to over liming, boron deficiency will occur.
where ‘x’ is the exchange site.
The freshly Al(OH)3 is then available for adsorption of boron. This reaction mechanism is involved for causing boron deficiency.
(v) Due to application of lime in excess, the incidence of diseases like scab in root crops will be increased.
All these effects can be reduced with the application of large amounts of organic manures like well rotten farm yard manure, green manure crops, compost, phosphorus, boron or a mixture of micronutrient fertilizers to the soil.