ADVERTISEMENTS:
In this article we will discuss about:- 1. Introduction to Salt Affected Soils 2. Classification of Salt Affected Soils 3. Salt Balance in Irrigated Lands 4. Reclamation of Salt Affected Soils 5. Acid Sulphate Soils.
Introduction to Salt Affected Soils:
High concentrations of certain salts when present in the soil cause soil and water management problems and ultimately affect crop production.
High concentration of soluble salts increases the solute suction and thus reduces the availability of soil water to plants. Some of them like sodium carbonate, soluble borates etc. could be toxic to plants.
ADVERTISEMENTS:
They could also be harmful indirectly as for example the rise of the soil pH by sodium carbonate makes nutrients such as phosphates, manganese and zinc becomes unavailable to plants. High concentrations of exchangeable sodium produce a soil structure breakdown which reduces permeability, aeration, infiltration rate and soil workability.
Salts are found in the soil-water and are also linked with the clay particles. There is a continuous interchange or exchange of salts as ions between the soil-water and clay complex to establish an equilibrium situation. The salts found in the soil solution, known as soluble salts can be removed from the soil by leaching and subsequent drainage.
The salts held by clay known as exchangeable salts, have to be exchanged before they could be removed by leaching. The soluble salts that occur in soils consist mostly of certain proportions of the cations of sodium, calcium, magnesium and potassium and the anions of chloride, sulphate, bicarbonate, carbonate and nitrate.
Classification of Salt Affected Soils:
Salt affected soils are classified based on their soluble salt concentration and exchangeable sodium content as these two are the important parameters affecting plant growth and soil condition. Since these parameters are dependent on the soil moisture content, a common moisture content viz., saturation of a disturbed soil sample in the laboratory is used for their determination. A soil sample is brought to saturation by stirring in distilled water until a characteristic end point is reached, where the soil glistens and flows slightly without free water standing on the surface.
ADVERTISEMENTS:
The solution (known as saturation extract) is extracted by placing the sample in a funnel with filter paper and applying suction. The total soluble salt concentration is estimated by measuring the electrical conductivity (EC) of the saturation extract.
An electrical conductivity meter is used for the purpose. It may be noted that the saturation extract is a diluted solution compared with soil- water at field capacity and the salt concentration is therefore different than at field capacity.
In case of highly soluble salts like chloride salts, EC is almost inversely proportional to the water content and its value at field capacity is taken to be twice of the value of saturation extract. As the preparation of the saturation extract is time consuming, soil water extracts 1:1 (100 gm of water per 100 gm of dry soil) is sometimes used for analysis. However, for comparison purposes the same amount of water should be used.
The total salt content of soil solutions or irrigation waters is usually expressed as electrical conductivity (EC) at 25 °C, parts per million (ppm) or milliequivalents per litre (meq/l) or unit used at present is decisiemens per m.
ADVERTISEMENTS:
The units of EC are mhos/cm. Mho is the reciprocal of resistivity expressed in ohms. The resistivity is the resistance in ohms of a conductor which is 1 cm long and has a cross-sectional area of 1 cm2. As the standard unit of EC, i.e., mhos/cm is a large unit so far as the solutions under consideration, it is customary to choose a smaller subunit for expressing the data.
The unit EC x 103 is called millimhos per cm and EC x 106 is called micromhos per cm. EC is usually expressed as mhos/m, which is referred to as siemens/m (S/m). The SI unit of EC is decisiemens per m (dS/m). It should be noted that 1 millimhos/cm = 1 dS/m. For example – if EC of a solution is 0.0006 mhos/cm, it is 0.6 millimhos/cm, or 600 micromhos/cm or 0.6 dS/m.
Parts per million is numerically equivalent to milligrams of the substance per litre. Milliequivalent per litre is the milliequivalent weight of anion or compound in a litre of solution. EC to meq/l is obtained using the relation –
for irrigation waters having EC in the range of 100 to 5000 micromhos per cm. Equations 16.1 and 16.2 are obtained by plotting these values for several salts commonly occurring in soils. Meq/l is converted to ppm by multiplying meq/l for each ion by its equivalent weight and adding them.
ADVERTISEMENTS:
The equivalent weight of an element or compound indicates its combining capacity with hydrogen. It is expressed as the weight in grams of the ion or compound that combines with or replaces one gram of hydrogen.
Equivalent weight is obtained by dividing the atomic weight with valency of the ion or compound. The milliequivalent weight is one thousand the part of the equivalent weight. Table 16.1 indicates the equivalent weights of some ions and salts relevant to problems of soil and water.
Example 1:
ADVERTISEMENTS:
8 gm of sodium chloride has been dissolved in 2 litres of water. Express the salt concentration in ppm, in mhos, millimhos, micromhos/cm, dS/m, and meq/litre.
Solution:
Exchangeable Sodium:
Every soil has certain capacity to adsorb the positively charged constituents of dissolved salts (cations), such as calcium, magnesium, sodium and potassium. This is known as the cation exchange capacity.
The different adsorbed cations can be exchanged for one another, for example – calcium by sodium depending upon their relative concentrations in the soil solution. Exchangeable sodium is the amount of adsorbed sodium in the soil, expressed in per cent of the exchange capacity, in milliequivalents per 100 grams of soil.
When the exchangeable sodium percentage becomes high (exceeds 10 to 20 per cent of the exchange capacity), the soil develops poor physical conditions (like low permeability to water and air, hard when dry and sticky when wet), high pH values (above 8.5) and associated nutritional disorders, resulting in reduction of crop growth. The poor physical conditions develop from the action of exchangeable sodium in dispersing the soil aggregates which in turn results in clogging of the soil pores.
Soluble sodium can be measured using a flame photometer and soluble calcium and magnesium by titration. Concentrations determined are those in the saturation extract and are expressed in milliequivalents/litre.
Fig. 16.2 is a nomogram for determining SAR of the saturation extract and for estimations corresponding ESP value.
The soil reaction, pH value is the negative logarithm of the hydrogen ion activity. It is used as an index of acidity or alkalinity of soils. It is determined either potentiometrically using a pH meter or by titration. Determinations of the different parameters mentioned above are given in detail in USSL (1969).
Using the parameters, a general classification of the salt affected soils is given in Table 16.2.
In saline soils the problem is one of high soluble salt concentration. Alkali soils have pH and soil structure problems while saline alkali soils have both high salt content and, sodium problems. They, however, are better structured than alkali soils.
As several factors influence the behaviour of these soils the above classification is to be taken only as a guide. Other soil factors particularly the texture has modifying influence. The effect of exchangeable sodium is influenced by the texture, type of clay minerals, potassium content and organic matter content. High ESP would be more problematic in fine textured soils than in coarse textured soils.
Salt Balance in Irrigated Lands:
In irrigated areas, particularly if the irrigation water contains considerable salts, it should be ensured that the salt accumulations in the rootzone are not beyond permissible limits for crops. The salt balance equation in crop rootzone can be written knowing the inputs and outputs of water and salt (Fig. 16.5).
This equation can be applied for any time period. In areas where the contribution from capillary rise, plant absorption and soil precipitation losses are negligible, Equation 16.8 becomes- 
These ratios are known as the Leaching Requirement (LR). It may be expressed as a fraction or per cent.
Leaching requirement is defined as the fraction of the irrigation water that must be leached through the rootzone in order to prevent the salt content exceeding a specified level. cp representing the electrical conductivity of the drainage water gives the salt tolerance of the plants.
If all the leaching is to be carried out by the irrigation water, the amount of irrigation water is given by the sum of the consumptive use and the drainage water i.e.,
This equation considers the contribution of rainfall towards crop water requirement but does not consider its leaching contribution. It also assumes that 100 per cent leaching efficiency which may not be always achievable. Where salt precipitation and removal of salts by crops are significant, the leaching quantity is to be determined from the original salt balance equation.
The above equation is satisfactory on an annual basis. In irrigation practice, it will not be possible to achieve salt balance on a monthly basis as the water requirements and irrigation water availabilities may differ in each month. It is necessary to examine any system on a shorter time basis to prevent high salt contents occurring particularly at crop sensitive periods.
Table 16.8 gives an example of how salinity can vary during a cropping season with different amounts of irrigation water. As indicated in case (a) to maintain constant salinity throughout the season, a high rate of water application is necessary.
If a constant amount of irrigation water is applied as in case (b), deep percolation is reduced and salt build up will result. When the deep percolation is kept constant as in case (c) some salt build up will take place.
A comparison of the three possible management practices indicate that to maintain constant salinity throughout the season require high peak irrigation quantity (320 mm) and also a high drain discharge (160 mm).
With constant irrigation applications; there is a high peak deep percolation (requiring drainage) and very high salinity level (in 3rd month) which may or may not be acceptable to the particular crop. With constant deep percolation, there is a high irrigation requirement and also high salt build up.
Reclamation of Salt Affected Soils:
Investigations about soil properties, water quality and causes of salt problems need to be carried out before reclamation procedures are initiated. In many of the salt affected soils, watertable is often present at shallow depth.
In such situations suitable drainage systems need to be installed in order to control the watertable depth. Leaching of salts is done by applying water and for these purpose sufficient quantities of water has to be available.
The reclamation measures for salt affected soils depend upon the type of the soil viz.:
I. Saline,
II. Alkali, or
III. Saline-alkali.
I. Saline Soils:
The methods recommended are:
1. For saline soils with efforescence of salts at the surface:
(i) Scraping of the surface salts.
(ii) Flushing with water to wash away the excess salts.
2. For soils with high concentration of soluble salts into great depth but with deep water table:
(i) Impounding rain or irrigation water for leaching out injurious salts to a safe limit inside, the soil.
(ii) Surface drains coupled with flushing to remove salts by surface and partly by subsurface drainage.
3. For soils with high concentration of soluble salts up to great depth but with high water table:
(i) Lowering of the water table either by pumping or subsurface drainage,
(ii) Subsurface drainage.
In the reclamation of saline soils the objective is to reduce the soluble salt concentration to acceptable limits. Leaching is done for this purpose.
In order to estimate the amount of water needed for leaching, a leaching curve for the particular soil is prepared.
The following variables are defined for the purpose:
EC0 = initial electrical conductivity,
ECeq = final equilibrium electrical conductivity,
ECe = electrical conductivity of soil after given depth of leaching water has passed through per unit depth of soil,
DW = depth of leaching water, and
DS = depth of soil.
Leaching Equations:
The movement of salts in the soil profile is a complicated process as it is influenced by factors like soil texture, nature of clay fractions, rate of water movement, water quality etc. Detailed approaches are given in van Hoorn and van Alphen (1994). An approach wherein the entire rootzone is considered as a unit is presented here.
When water percolates down the rootzone, a fraction of the water mixes with the soil solution fully and displaces the salt while the other fraction is not effective in displacing the salts. This fraction of the water mixing with the soil solution is defined as the leaching efficiency coefficient (f).
After the first application of the irrigation water, it can be assumed that the soil water content will be nearly at field capacity.
As the leaching efficiency coefficient is generally variable, the concept of leaching curve is used in determining the amount of water required for leaching.
Leaching Techniques:
Leaching of saline soils is done by ponding water on the surface for long periods. Water moving under saturated conditions leaches the salts down the profile. Studies on intermittent leaching indicated that leaching could be effective even with intermittent water application.
This method is well suited especially for clay soils forming cracks. Salts moved towards the cracks due to capillary movement are washed down with the next water application. In intermittent application like in sprinklers salts are transported in the unsaturated phase.
Compared to continuous ponding intermittent application needs less water. Also intermittent applications will not help building up watertable unlike ponding. Watertables approaching the soil surface could again bring up the salts.
To prepare the leaching curve, an infiltration test is conducted in the same manner but in addition the soil is sampled before the test and again in the inner cylinder after the water has infiltrated to depths of 50 and 100 cm and salt content of the samples determined.
From the initial moisture content of the soil, the depth of water needed to bring the soil to field capacity is calculated and the same is substracted from the total depth infiltrated in order to find the quantity of leaching water. The soil will tend to reach an equilibrium salt content.
The top soil will get to this state during the test and this value (ECeq) is to be determined. The results are plotted as the leaching curve (Fig. 16.6). It should be remembered that this curve will be valid only for the particular soil and the particular quality of the water used for leaching.
Example 2:
It is required to reduce the electrical conductivity of the top 60 cm of soil from 20 mmhos/cm to 8 mmhos/cm. Given ECeq = 3 mmhos/cm, evaporation = 1 cm/day and water required to raise 60 cm to field capacity from initial moisture content is 20 cm find the depth of water needed for leaching.
Solution:
II. Alkali Soils:
Reclamation of these soils is achieved by:
(1) Treating with chemical amendments like gypsum, sulphur, etc.,
(2) Adding organic material such as farm yard manure, crop residues and green manuring,
(3) Leaching the products of reaction after amendments are added, and
(4) Deep ploughing to break any hard pan for improving drainage.
The objective in alkali soils is to reduce the exchangeable sodium percentage and remove the released sodium salts. To replace the sodium in the clay complex, chemicals such as gypsum (CaS4O.2H2O) and sulphur are added to increase the concentration of soluble calcium so that it can replace the exchangeable sodium.
The difficulty with alkali soils is that they are highly impermeable and as such proper mixing of the amendments and subsequent leaching are very difficult. Permeability is temporarily improved by cultivation. Because of the low permeability rates installation of subsurface drains in these soils are not likely to be successful. In order to effect leaching, it is desirable to install temporary shallow drains.
The choice of the chemical amendments depends upon the soil characteristics and on the availability and cost of the material in that particular area.
The amount of gypsum needed is calculated as illustrated in the following example. Suppose 0 to 100 cm layer of an alkali soil contains 4 meq of exchangeable sodium per 100 gm and has a cation exchange capacity of 10 meq per 100 gm.
The exchangeable sodium percentage is 40. Suppose it is desired to reduce this to about 10. This will require the replacement of 3 meq of exchangeable sodium per 100 gm. Assuming quantitative replacement, it will be necessary to apply the amendment at the rate of 3 meq per 100 gm of soil.
Table 16.9 provides the amount of gypsum to be applied for this purpose as 41.8 tonnes per hectare, if 1 m depth of soil is to be reclaimed.
Some of the other amendments used and their equivalence to gypsum are given in Table 16.10.
USSL (1969) suggest that the values of gypsum requirement given in Table 16.9 be multiplied by a factor 1.25 to compensate for the lack of quantitative replacement. They also suggest a method known as Schoonover’s method for estimating the gypsum requirements.
Field experience has however- shown that gypsum requirements calculated by the above methods are on higher side of the actual needs. It could also be economically prohibitive to apply such large quantities of gypsum.
The quantity of gypsum needed to reclaim a soil to grow crops depends upon the crops to be grown, nature of soil and its stage of deterioration. Abrol et al. (1973) observed that for alkali soils occurring in the Indo-Gangetic alluvial plains a fair good correlation exists between pH (1 : 2 soil water suspension) and exchangeable sodium percentage and therefore pH and the gypsum requirements of the soil.
The gypsum requirements given by them are shown in Fig. 16.7. The requirements are for reclaiming top 30 cm of soil. The relationships given in Fig. 16.7 are approximate and not valid for all other soils. They also observed that soil with pH lower than 9.0 (1 : 2 soil water suspension) will seldom need gypsum for growing crops like paddy and barley. However, if the pH is above 10.0, no crop is likely to grow unless gypsum is applied.
The application of gypsum needs to be done with caution using the research information available for the situation under consideration. Since gypsum has low solubility, application of large quantities in a single dose is not desirable.
For reclamation of sodic soils in India, it was recommended that gypsum be applied on a well prepared soil (prior to the onset of the rainy season). Incorporation into the soil may not be necessary.
III. Saline Alkali Soils:
These soils are comparatively easier to reclaim than alkali soils. In certain cases these soils can be reclaimed by leaching alone. Leaching tests should be conducted to ascertain whether additions of amendments are necessary or not.
When amendments are needed, it is necessary to the amendments before leaching the soluble salts or otherwise soil structure may deteriorate. A simple leaching test consists of observing the infiltration rate during a period of 7 to 14 days.
If the infiltration rate is similar to the minimum rate during the first few hours then there is a good indication that little damage will result from leaching. If there is deterioration, chemical amendments are necessary.
After reclamation of the salt affected soils it is necessary to prevent their resalinization. This is achieved by maintaining a salt balance, drainage and controlling the depth of the watertable.
Acid Sulphate Soils:
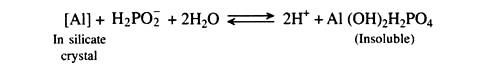
Acid sulphate soils are soils with a pH below 4 caused by sulphuric acid formed by oxidation of pyrite (FeS2). These are formed in marine or brackish sediments. Potential acid sulphate soils may be neutral or slightly acidic in the field. With drainage, the soils become strongly acidic, which directly affects the growth of plants as a result of iron and aluminium toxicity and also indirectly decreases the availability of phosphorus and other nutrients.
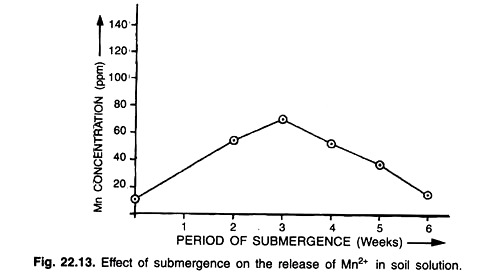
Acid sulphate soils occur in several countries of the Asian region particularly in India, Thailand, Indonesia and Malaysia. Acid sulphate soils to some extent are being successfully used for rice cultivation in Thailand. It has been generally observed that when these soils are flooded, the pH rises and if submergence is continued the pH increases sufficiently so that aluminum toxicity is eliminated and iron toxicity is minimized.
The steps in using these soils for lowland rice cultivation are as follows:
1. Leaching and drainage to remove soluble salts.
2. Submergence to increase the pH value.
3. Liming to increase the pH value of the soil.
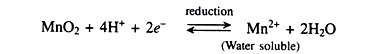
4. Addition of manganese dioxide (MnO2) for improving nutrient availably.
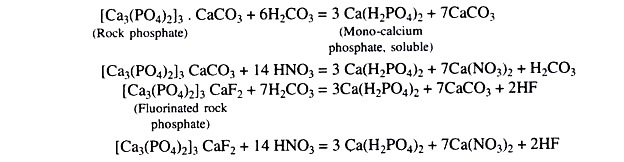
5. Nitrogen, phosphorus and potassium application including application of rock phosphate.
6. Use of resistant crop varieties.
Dent (1986) gives detailed description of the acid sulphate soils and present management strategies.
Utilization of Salt Affected Soils without Reclamation:
In situations where it is uneconomical to reclaim salt affected soils, it is desirable to use these areas for suitable vegetative plantings. Salt tolerant trees and grasses which could have some economic value need to be planted in such areas.