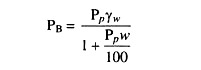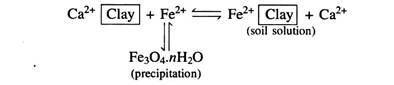ADVERTISEMENTS:
After reading this article you will learn about:- 1. Definition of Submerged Soils 2. Kinds of Submerged Soil 3. Importance 4. Properties.
Definition of Submerged Soils:
Submerged soils are soils that are saturated with water for a sufficiently long time in a year to give the soil the following distinctive gley horizons resulting from oxidation-reduction processes:
(i) A partially oxidized ‘A’ horizon high in organic matter.
ADVERTISEMENTS:
(ii) A mottled zone in which oxidation and reduction alternate and
(iii) A permanently reduced zone which is bluish green in colour.
The soil is intermittently saturated with water, oxidation of organic matter is slow and it accumulated in the “A” horizon. In the second horizon, Fe and Mn are deposited as rusty mottles or streaks if the diffusion of O2 into the soil is slow, if the diffusion is rapid, they are deposited as concretions.
Kinds of Submerged Soil:
There are different kinds of submerged soil, of which
ADVERTISEMENTS:
(i) continuous submerged soils and
(ii) alternate submerged soils are important for rice cultivation.
Continuous submergence at a static 2.5-7.5 cm depth provides the potential to produce optimum rice yields. Generally continuous submergence at 15 cm depth or more has the potential to produce yield similar to those at 2.5 cm water depth. However, in some dry seasons a 15 cm depth or more may reduce grain yield.
Importance of Submerged Soils:
Rice is the only major food crop that can be grown under various degrees of submergence.
ADVERTISEMENTS:
Submerged conditions exhibit the following characteristics:
(a) Greater amount of soil solution,
(b) Reduced oxygen level,
(c) Reduced aerobic microbial activity and
ADVERTISEMENTS:
(d) An altered chemical status of the soil.
The rice plant absorbs nutrients and grows under these specific complex conditions.
Generally systems of rice cultivation in India and especially in West Bengal can be classified into two major types:
(i) Semi-dry systems—where rice is grown primarily as a rainfed crop especially in uplands. The yields under such conditions are normally low,
ADVERTISEMENTS:
(ii) Wet-system, practiced in low lands or where irrigation is available.
In this system of rice cultivation, the production is very high. Soil submergence for rice cultivations is thus essential. Because as a result of soil submergence a variety of changes like physical, chemical, electrochemical and biological properties are modified of which majority of soil prosperities is suitable for rice cultivation.
Properties of Submerged Soil:
There are various changes like physical (depletion of oxygen, accumulation of carbon dioxide, compaction, bulk density, puddling, gaseous exchange and movement of water etc.), Chemical (soil reduction and transformation of different nutrient elements etc.), electro-chemical (soil pH, specific conductance and redox potential, Eh etc.) and biological properties (decomposition of organic matter mineralization and immobilization processes etc.) of soils that are strongly influenced by the soil submergence.
A. Physical Properties:
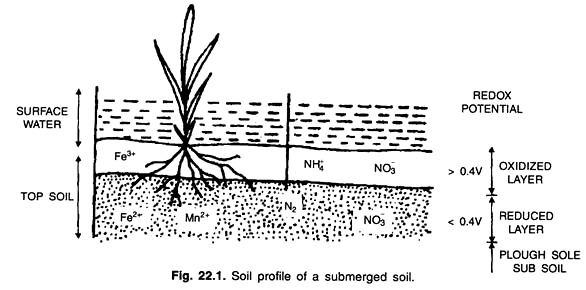
1. Diffusion of Molecular Oxygen and Development of Aerobic Anaerobic Layer:
When a soil is submerged, water replaces the air in the pore spaces. Except in a thin layer at the soil surface, and sometimes a layer below the plough sole, most soil layers are virtually oxygen-free within a few hours after submergence. The oxygen-diffusion in the water layer above the soil is very slow and the rate of O2 consumption is reduced soil is high.
Because of this high demand of O2 in submerged soil and slow O2 supply through water, the soil is practically devoid of oxygen. This rapid depletion of O2 takes place within a day or so of submergence. Some O2 trapped in blocked pore spaces is rapidly utilized by facultative anaerobic organisms.
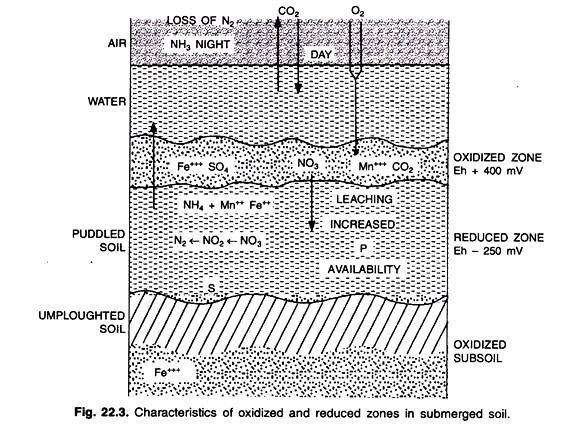
The greater potential consumption of O2 as compared to the available supply through the flood water results in two distinctly different layers being formed in a submerged soil: an oxidized or aerobic surface layer where O2 is present and a reduced or anaerobic layer in which no free O2 is present (Fig. 22.1)
The thickness of the aerobic surface layer is determined by the ratio of O2 supply from the atmosphere to the O2 consumption in the soil.
Oxygen reaching the submerged soil surface is utilized in several ways:
(a) As an electron acceptor by soil micro-organisms in their cell respiration,
(b) In the chemical oxidation of ferrous iron (Fe2+) and manganous manganese (Mn2+) and
(c) For biological oxidation, such as oxidation of NH4+ —N to NO3-—N and S2- and S° to SO42-.
If the demand of O2 exceeds the supply, a thin oxidised surface layer, an mm thick or so, is formed. A soil rich in organic matter or readily decomposable organic residues has a thin oxidized layer. In soil poor in organic matter as in laterites where percolation rates are high, the oxidized layer may be several cm thick.
2. Aeration Status of a Submerged Soil:
Immediately after submergence, the normal process of gaseous exchange between soil and air is restricted. The magnitude of this effect is evident from the relative values in air and in water of the diffusion co-efficient D, in the following equation,
V = – aD (T/T0)2 . dp/dl
where, V = volume of gas, C.C cm2 sec-1
a = porosity factor
dp/dl = pressure gradient
T = absolute temperature
For oxygen the ratio D water/D air is of the magnitude of 10-4. The changes in porosity factor and pressure gradient are insignificant compared with the diffusion coefficient. Thus the entry of O2 and other atmospheric gases in the soil is severely restricted.
The escape of soil gases by diffusion is also affected to the same degree. The net result is that the concentration of O2 in the soil is reduced to a very low value, while that of soil gases, notably CO2 is increased, especially if conditions are favourable for biological activity.
3. Accumulation of Carbon dioxide:
Soil gases like CO2 and methane (CH4) accumulate due to submergence, and also may escape as bubbles if pressure builds up. It has been recorded that during the first three weeks submergence some soils may generate CO2upto2.5 t ha-1.
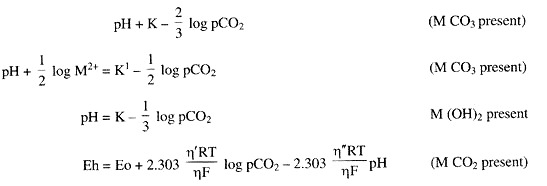
Due to presence of large amounts of carbonic acid (H2CO3) the chemical equilibrium in soil-water system containing high amount of Ca2+, Mg2+, Fe2+ and Mn2+ affects. The partial pressures of CO2 will determine the solubility of all these cations.
Some chemical equilibria are given below:
According to these equations pCO2 in a reduced soil will determine its pH, Eh, solubility of cations and thereby affect the specific conductance and cation exchange reactions. The increase in pCO2 on submergence is due to the accumulation of CO2 initially produced by aerobic respiration followed by anaerobic decomposition of organic matter. Hence, the pCO2 in soils high in organic matter is higher.
The higher pCO2 in acid soils than in neutral soils can be explained by the presence of higher proportion of H2CO3 as follows:
(H2CO3)/(HCO3–) = (H+)/K
The decrease in pCO2 after one to four weeks of submergence may be due to the following reasons:
(i) Escape from the soil,
(ii) Diluting effect of methane (CH4) produced in the later period of organic matter decomposition,
(iii) Reduction of CO2 leaching losses and removal of CO2 as insoluble carbonates.
The rapid decline in the concentration of CO2 in soils high in iron and manganese suggests is follows:
Fe2+ + CO32-→ FeCO3
Mn2 + CO32-→ MnCO3
4. Soil Compaction:
In compaction, soil solids are re-arranged with compression of liquid and gaseous phases accompanied by volume change. Soil compaction affects the water retention characteristics, water intake rates, and gaseous exchange.
In compacted soil, bulk density, micro-voids, thermal conductivity and diffusivity and nutrient mobility increases and on the other hand, macro-voids, hydraulic conductivity and water intake rates decrease. Generally medium textured soils are most susceptible to compaction.
Compaction is the increase in soil density caused by dynamic loading. During compaction soil particles move to a closer state to contact and bulk density, porosity etc. change. The intensity of compaction largely depends on soil moisture, comp-active energy, nature of the soil and the amount of manipulation.
The state of compaction affects the soil-air-water- temperature relationship profoundly and determines the physical, chemical and biological properties of soil.
Dry pulverized soil cannot be compacted to high density because of the incompressible nature of soil particles and high internal friction. But as a soil moisture content increases and the thickness of the water films around soil particles increases, the cohesion among particles decreases allowing them to slide over each other and the soil can thus be compacted readily to a greater density.
(i) Bulk Density:
When soil is compacted to such a degree that all voids (pore spaces) are filled with water, no air voids (spaces) are present, and no soil water is expelled from the voids, the soil is saturated and its bulk density is maximum.
So the bulk density of saturated soil can be calculated from the following equation:
where, PB = Bulk density
PP = Particle density
w = Water content and
yw = Unit weight of water
(ii) Water Retention and Transmission:
Both water retention and transmission are affected as compaction changes pore-size distribution and fabric geometry. In compacted soils the water retained at low suction and saturation decreases but more water is retained at high suction. Thus k, compaction increases plant-available water in a soil. But because non-capillary pore space conducts most of the water by decreasing macroporespaces.
(iii) Gaseous Exchange:
Gaseous exchange within the soil and between soil and atmosphere is primarily a function of air-filled pores, or aeration porosity. By destroying air-filled pore space, soil compaction limits gas transfer in a soil.
So compaction may modify the gas composition of soil if porosities are reduced sufficiently.
(iv) Soil Temperature:
Soil temperature is important in determining the energetics of soil- plant interactions. Compaction affects the thermal characteristics of soil by modifying the fabric geometry, soil-water relations etc. As the thermal conductivity of soil particles is higher than that of air, increased density decreases the volume of gases and increases the thermal contact between the soil particles.
As a result thermal conductivity increases. Thermal diffusivity is the ratio of thermal conductivity to volumetric heat capacity, as it increases, heat flow increases. Thermal diffusivity increases as degree of saturation and compaction increase.
Therefore, it may be concluded that soil compaction cuts down percolation losses and reduces the water requirement of rice, appears to be a more practical and economical tillage practice than puddling for increasing rice yield and water use efficiency.
5. Puddling:
Puddling refers to breaking down soil aggregates at near saturation into ultimate soil particles. The mechanical reduction in the apparent specific volume of soil is found due to puddling. Only soils with more than 20 per cent of clay particles are prone to puddling.
Puddling and subsequent flooding differentiate low and rice soils chemically and pedologically from other arable soils. An important difference between a dry land and puddled low land soil system.
Puddling, an intensive wet land cultivation, breaks the natural aggregates to finer fractions. It decreases the apparent specific volume and hydraulic conductivity, creates an anaerobic environment and affects redox (Eh) and pH values.
Influence of Puddling on Soil Properties:
Puddling influences physical, chemical and biological soil properties which in turn influence rice growth. Puddling has both short and long term effects on soil and rice growth.
A. Short Term Effects:
(i) Soil Structure:
Puddling destroys aggregates and peds. Wetting dry soils cause uneven swelling in aggregates and explosion of trapped air. The aggregates are slaked. Aggregates, depending on their stability, will be partially or completely destroyed “if they are submerged and objected to repeated” ploughing, harrowing or other puddling.
The puddled layer is neither structurally nor chemically uniform, but there is little information on stratification within puddled soil.
(ii) Bulk Density and Soil Strength:
The effect of puddling on bulk density depends on soil aggregation before puddling. If puddling produces a parallel, closely packed structure from a well-aggregated open structure, bulk density increases. But puddling can also produce a more open structure, and hence decrease bulk density.
Puddling decreases the shear strength of the surface layers. In general, shear strength decreases with moisture content and increases with bulk density.
(iii) Soil Porosity:
In a puddled soil, individual clay particles or particle clusters are in parallel rows within water-saturated capillary pores. The effect of puddling on porosity depends on the soil particle orientation in the puddled layer. If a more open structure results from puddling, total porosity will increase.
(iv) Gas Exchange:
In a submerged pudled soil, gas exchange, especially of O2 between soil and atmosphere is strictly restricted. Oxygen concentration decreases and carbon dioxide concentration increases.
(v) Water Retention and Movement:
Water retention in puddled soils always exceeds that in un-puddled soils. Puddled soils dry more slowly than un-puddled soils, probably because the higher unsaturated hydraulic conductivity of puddled soils can keep surface soil wet during evaporation by supplying water from lower layers.
B. Long Term Effects:
Long-term puddling forms a hardpan in the subsoil below the puddled layer. Subsurface hardpans develop from physical compaction and precipitation of Fe, Mn and Si. Chemically cemented pans are formed in the oxidised subsoil, usually 15 to 20 cm deep, by precipitation of Fe, Mn and Si from upper reduced soil layers.
Continuously submerged soils have excessively reduced conditions, Fe— and Mn—pans—form very slowly. Ferrolysis is another long-term effect of puddling that may lower soil productivity.
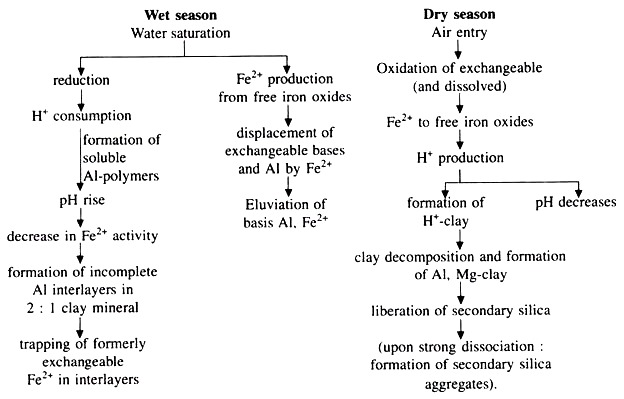
Ferrolysis:
Ferrolysis is the process of clay decomposition and transformation under the influence of periodic reduction of iron oxides to ferrous ions (Fe2+). Ferrolysis comprises various component processes. Some operating in reduced conditions (wet season) alternating with other operating in oxidising conditions (dry season).
The schematic representation of ferrolysis during wet and dry seasons is depicted below:
Ferrolysis is not desirable for rice crop growth. Ferrolysis can be stopped in different ways:
1. By eliminating the oxidation phase.
2. By eliminating the reduction phase. This can be done by rapid leaching which would entail great losses of nitrogen and other nutrients or by shifting to up land rice.
3. By raising the dry season (oxidised) pH by liming.
4. By replacing the basic cations lost ‘either through fertilizing or liming or base rich irrigation water etc.
All these measures are partially effective, since clay decomposition takes place rapidly in the first few days after air-entry and since the re-supply and even distribution of bases is generally a slower process.
B. Electro-Chemical Changes in Submerged Soils:
The main electro-chemical changes in submerged soils are:
(i) Decrease in redox potential (Eh).
(ii) Increase in pH of acid soil and decrease in pH of alkaline soils,
(iii) Increase in specific conductance,
(i) Redox Potential (Eh) of Submerged Soil:
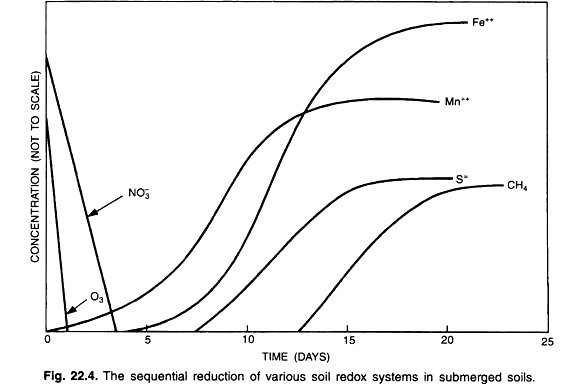
When a soil is submerged the reduction of soil takes place and the reduced soil contains reduced counter parts of NO3–, SO42-, Mn4+, Fe3+ and CO2: NH4+, H2S, Mn2+, Fe2+ and CH4. The reduction of different soil components takes place in a sequential form; nitrates are reduced first followed by manganic (Mn4+) and so on.
Oxidation-reduction is a chemical reaction in which electrons are transferred from a donor to an acceptor. The electron donor losses electrons and increases its oxidation number or is oxidised, the acceptor gains electrons and decreases its oxidation number or is reduced. The source of electrons for biological reductions is organic matter.
The driving force of a chemical reaction is the tendency of the free energy of the system to decrease until, at equilibrium, the sum of the free energies of the products equals that of the remaining reactants. In a reversible oxidation reduction reaction, this force can be measured in calories or in volts. The change in free energy, ∆G, for the reduction,
where (Red) and (Ox) are the activities of the reduced and oxidised species and ∆G° is the free energy change when the activities are unity. Converting calories to volts using the relationship,
From the following equation,
in which E is the voltage of the reaction, E0 is the voltage when (Ox) and (Red) are each unity, and F is the Faraday constant in heat units. If E is measured against the standard hydrogen electrode, it is denoted by Eh. Equation (2) then becomes.
Eh is a quantitative measure of the tendency of a given system to oxidize or reduce substances. Eh is positive (+ve) and high in strongly oxidising systems; it is negative (-ve) and low in strongly reducing systems. There is no neutral point as in pH. Any chemical reaction which involves the exchange of electrons will be influenced by redox potential (Eh).
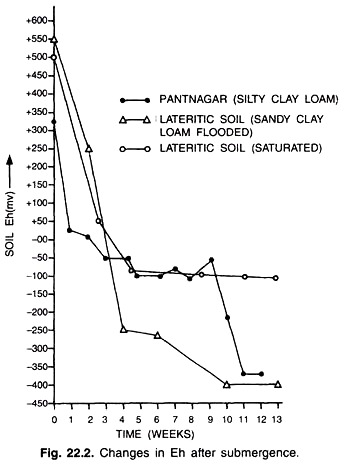
When a soil (aerobic) is submerged, a sharp drop in the potential will observe and within a few weeks negative (-ve) potentials are observed in most of the soils. The submerged soils may exhibit potentials as low as -300 to -400 mV depending upon the type of soil organic matter, Fe and Mn content, a pH and temperature i.e. presence of poisoning system and bacterial potentials (Fig 22.2).
The study of redox potential d will help us in better understanding w of the rice environment which influences the growth, nutrition and yield. Soil reduction per se is not harmful for the growth of rice unless the value of redox potentials (Eh) is too low for sulphide formation. However, redox potential influences the rice cultivation under three conditions depending on the soil and water management practices:
(i) Highly oxidised state initially as in uplands where NH4—N may be oxidised to NO3—N and may leach or denitrify. The oxidation of organic residues leads to the liberation of CO2, NO3, and SO4 etc. The availability of iron, manganese and phosphorus will be very limited since they are present as their oxidised forms (Fe3+ and Mn4+) and phosphorus as insoluble iron and manganese phosphates. The pH may be too low resulting aluminium toxicity.
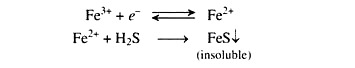
(ii) Healthy reduced state where NH4—N is present in large amounts and is absorbed on soil colloids resulting very little leaching and denitrification losses. The availability of iron will increase due to reduction of Fe3+ to Fe2+. Generally H2S will not form at this stage, but if it forms, it will precipitate as FeS (iron sulphide).
In this state of soil reduction, pH is around about 6.5 which is optimum for the availability of all the macro- and micro-nutrients.
(iii) Highly reduced toxic state results due to prolonged submergence.
In this situation, there may be appearance of iron toxicity because of its maximum concentration. Sulphide (S2-) ions may accumulate and cause sulphide toxicity to rice roots. The generation of organic acids (butyric acid, propionic acid etc.), ethylene, mercaptans, organic sulphides etc. will found which will create problem to the rice plant (Fig. 22.3).
Therefore, the low redox potential (Eh) resulting from soil submergence, influences the rice growth which are as follows:
1. A low Eh harms germination and seedling emergence but not growth of the well-established plant,
2. Low Eh destroys NO3– —N but favours NH4+ —N accumulation and nitrogen fixation and hence results net increase in the soil nitrogen regime,
3. Low redox potential benefits rice by increasing the availability of N, P, Si, Fe, Mn, and Mo.
4. It causes harmful effect to rice by decreasing the availability of S, Cu and Zn.
Sequential Reduction of Oxidation-Reduction Systems in Submerged Soils:
After the disappearance of O2 in submerged soils, the need for electron acceptors by facultative and obligate anaerobic micro-organisms results in the reduction of several oxidised soil components. In the presence of a readily available energy sources, microbes utilize several of the oxidised soil components and reduced the oxidation number of the oxidised atom.
Some of the oxidised soil components that undergo reduction after O2 is depleted are reduced sequentially as follows:
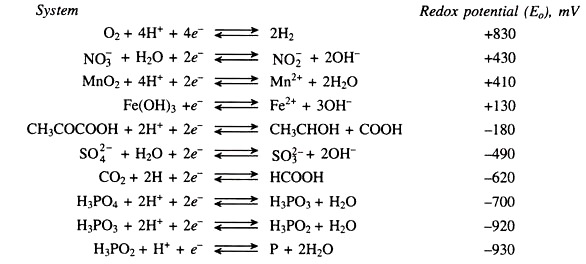
The general sequence of reduction is shown is Fig. 22.4.
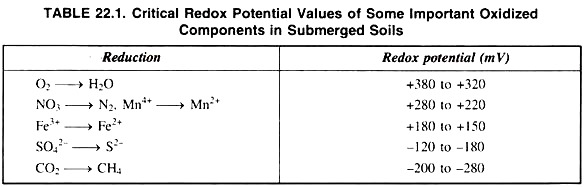
In-spite of an overlap in the reduction of such various redox systems, knowledge of the approximate critical redox potential at which these systems become unstable is useful for characterizing the intensity of anaerobiosis in soils. So critical redox potential values of some important oxidised components are shown in table 22.1.
pE Concept:
It is more logical and convenient to use pE instead of Eh in the study of redox equilibria. The common reagent in redox equilibria is the electron. Just as pH is a measure of proton (H+) activity, so in pE, the negative logarithm of the electron activity, a measure of electron activity. It can be written as,
pE = -log (e)
= Eh/2.303 RTF-1
Or pE = Eh/0.0591
and pE0 = Eo/0.0591 at 25°C
So, for the determination of pE value of redox potential (Eh) divided by 0.0591 will give the pE value.
(ii) Soil pH:
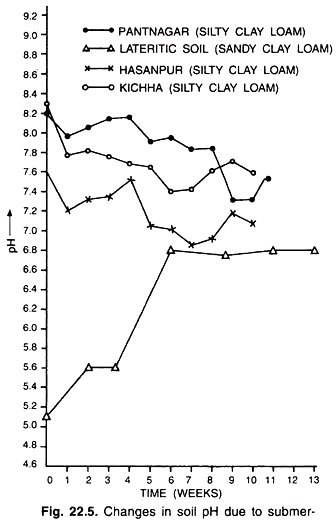
When an aerobic soil is submerged, its pH decreases during the first few days reaches a minimum, and then decreases asymptotically to a fairly stable value of 6.7-7.2 a few weeks later.
The overall effect of submergence is to increase the pH of acid soils and to decrease the pH of alkaline soils. Thus submergence makes the pH values of acid soils (except those low in iron) and alkaline soils converge to neutral soil pH 7.0 (Fig. 22.5).
The increase in pH is acid soils may be due to the reduction of iron (Fe3+) and manganese (Mn4+) and also due to the presence of organic matter that enhance the process of soil reduction. Since the reduction process removes hydrogen ions (H+) from the solution, as reduction proceeds so the pH of the acid soil rises.
Soils, however, contain CO2 dissolved in the soil solution, and if the conditions are suitable, either ferrous carbonate (FeCO3) will be precipitated of the CO2 in the soil solution, through its effect on pH, will modify the concentration of ferrous ions (Fe2+) in equilibrium with ferroso-ferric hydroxides [Fe3(OH)8].
On the other hand, the decrease in pH of alkali and calcareous soils may be due to the release of sufficient amount of CO2 during decomposition of organic matter. The increased concentration of CO2 produces hydrogen ions (H+) reacting with soil water and thereby decreases the soil pH.
The pH of submerged alkali and calcareous soils are generally governed by the system Na2CO3—H2O—CO2 and CaCO3—H2O—CO2 respectively.
Soils high in organic matter and reducible iron attain a pH of about 6.5 within a few weeks of submergence. Acid soils low in organic matter or active iron slowly attain pH values which are less than 6.5.
In fact, acid sulphate soils low in iron may not attain a pH of more than 5 even after months of submergence. Organic matter magnifies the decrease in pH of sodic and cal. careous soils. Low temperature or the presence of nitruate (NO3–) retards the increase in pH.
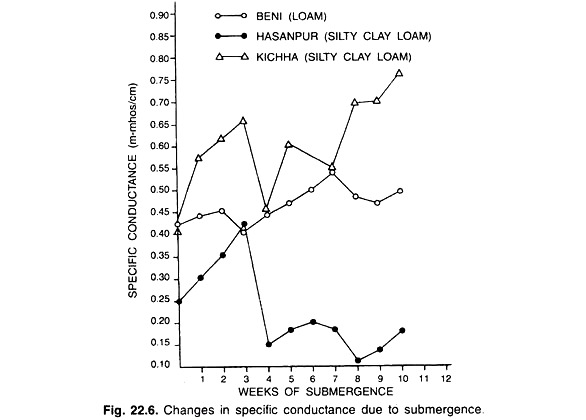
(iii) Specific conductance:
The specific conductance of the solution of most soils increases after submergence; attain a maximum, and declines to a fairly stable value, which varies with the nature and properties of the soil (Fig. 22.6).
The increase in conductance during the first few weeks of submergence is due to the release of Fe2+ and Mn2+ from the insoluble Fe3+ and Mn4+ oxide hydrates, the accumulation of NH4+, HCO3– and RCOO–, and in case of calcareous soils, the dissolution of CaCO3, MgCO3 by CO2 and organic acids.
Besides, the increase in the concentration of calcium and magnesium may be due to the displacement of ions, especially cations from the soil colloids by the following reactions:
The decline after the maximum increase is mainly due to the precipitation of Fe2+ as Fe3O4nH2O and Mn2+ as MnCO3. The decrease in conductance of calcareous soils is caused by the fall in partial pressure of CO2(pCO2). and the decomposition of organic acids.
The kinetics of specific conductance varies widely with the soil properties. The strongly acid lateritic soils show a steep increase in specific conductance during first week of submergence. Soils high in organic matter attain higher peaks.
In soils in which reduction proceeds slowly specific conductance increases gradually to a level of 1-2 m mhos cm-1 and thereafter declines slowly. The calcareous soil low in organic matter shows no marked change in specific conductance.