ADVERTISEMENTS:
After reading this article you will learn about the transformation of important nutrient elements in submerged soils:- 1. Nitrogen 2. Phosphorus 3. Potassium 4. Sulphur 5. Iron 6. Manganese 7. Zinc 8. Copper 9. Boron and Molybdenum.
Nutrient Element # 1. Nitrogen:
Nitrogen occurs in soils mainly as complex organic substances, ammonia, molecular nitrogen, nitrite and nitrate. The transformations of nitrogen are largely micro-biological inter-conversions regulated by the physical and chemical environment of the soil.
The main inter-conversions are shown below:
In submerged soils, the main transformations are accumulation of ammonia, volatilization loss of ammonia, denitrification, nitrogen fixation and leaching losses of nitrogen. These transformations have an important bearing on the nutrition of rice. It is evident that nitrogen is deficient in rice soils because of conditions favourable for rapid transformations and losses of nitrogen from the soil.
Mineralization of Nitrogen and Accumulation of Ammonia:
In aerated soils NO3– is the inorganic form and all of the nitrogen reactions that follow the composition of organic matter proceed towards the production of NO3–. Thus organic form of nitrogen undergoes mineralization to NH4+, oxidation of NH4+ to NO2– and oxidation of NO2– to NO3–.
In aerobic soils
But in anaerobic soils the absence of O2 inhibits the activity of the Nitrosomonas micro-organisms that oxidises NH4+ and therefore, nitrogen mineralization stops at the NH4+ form.
In submerged soil
The accumulation of ammonia in submerged soils is, therefore, a good index of the capacity of a soil to meet up the demand for nitrogen to the rice crop. The transformation of nitrogen occurs in the aerobic and anaerobic layers of a submerged soil.
ADVERTISEMENTS:
In the aerobic surface layer, conditions are similar to those of a well-drained soil and nitrogen mineralization proceeds to the NO3– form. The presence of an aerobic layer above the anaerobic layer is the major cause of instability of nitrogen in submerged soils and results in considerable loss of nitrogen through nitrification-denitrification reactions.
Nitrate is stable and not subject to denitrification as long as it remains in the surface aerobic layer, but it readily diffuses downward into the anaerobic layer and undergoes denitrification as a result of a gradient in the NO3– concentration between the aerobic layer and the anaerobic layer.
This process can proceed as long as NO3– is formed in the aerobic layer, and that can readily happen if there is a sources of NH4+ in the aerobic layer that can be nitrified (NO3–). The removal of NH4+ in that layer by nitrification creates a concentration gradient that causes NH4+ to diffuse upward form the anaerobic layer.
The overall sequential transformations of organic nitrogen to elemental nitrogen in submerged soils are depicted in Fig. 22.7.
Besides, the overall transformations of nitrogen in submerged soils are shown in Fig. 22.8.
Nutrient Element # 2. Phosphorus:
Soil submergence is known to influence the transformation and availability of both native and applied phosphorus. Phosphorus is not directly involved in oxidation-reduction reactions in redox potential range encountered in submerged soils, but because of its reactivity with a number of redox elements its behaviour is significantly affected by waterlogging. On submergence, the availability of native as well as applied phosphorus increases in the soil. The phosphorus transformation is also known to be associated with pH changes in submerged soils.
Phosphate chemistry in submerged soils is linked to iron chemistry and conditions that increase solubility of iron in the soil usually increase phosphorus solubility. Phosphate is chemically associated with iron in two major forms in an aerobic soil (oxidised soil)—as insoluble iron phosphate compounds such as strengite (FePO4.2H2O) and as the more soluble phosphate compounds like calcium and magnesium phosphates that are co-precipitated with insoluble ferric oxy-hydroxides.
ADVERTISEMENTS:
When an aerobic soil is submerged the concentration of available phosphorus initially increased and thereafter declines with the period of submergence. However, the magnitude of initial increase and decrease in the later period of submergence depends on the soil properties (Fig. 22.9).
The increase in phosphorus availability on submergence may be attributed to the following mechanisms:
(i) Release of P from the mineralization of organic residues,
(ii) Reduction of FePO4.2H2O to the more soluble Fe3(PO4)2, 8H2O and increase in solubility of FePO4.2H2O and AlPO4.2H2O caused by the increase in pH coupled with the reduction of acid soils.
(iii) Release of co- precipitated or occluded phosphorus due to reduction of ferric oxy-hydroxide.
(iv) Displacement of P from ferric and aluminium phosphates by organic anions
(v) Increase in solubility of calcium phosphates (CaHPO4.2H2O, Ca4H (PO4)3.3H2O, Ca10 (PO4)6 (OH)2, Ca10 (PO4)6 CO3 and Ca10 (PO4)6F2) associated with the decrease in pH caused by the liberation of CO2 in the calcareous soils.
(vi) The release of P due to anion exchange reactions between clay and phosphate or organic anions and phosphate. The decrease in the concentration of available P at the later period of submergence may be due to the fixation (through adsorption) of released phosphorus by clay colloids (kaolinite, montmorillonite and hydrous oxides of Fe and Al).
In addition, the decreased concentration of phosphorus may also be due to the decreased solubility of phosphorus associated with calcium. The formation of insoluble iron oxide and hydroxides sometimes results low pH phosphate concentration of soil 5 solution. Under submerged conditions (reduced), however, maximum phosphate solubility occurs under low pH conditions (Fig. 22.10).
Therefore, it may be concluded that more phosphorus is released from the soil to the soil solution under submerged conditions (reduced) than that of upland soil conditions (oxidised) if the solution is initially low in phosphorus as it is found in low land rice soils. The transformations of P under alternate wetting (submergence) and drying will not be similar to that of continuous submergence. Alternate wetting and drying reduces the availability of P in Al-P fraction and that increase in Fe-P fraction.
Nutrient Element # 3. Potassium:
In soils two important parameters influence the availability of K to plants. These are (i) the intensity factor (I), which is the concentration of an element in the soil solution and (ii) the capacity factor (Q), which is the ability of solid phases (soil) to replenish that element as it is depleted from solution. As plants remove K+ ions from the soil solution, the concentration of K+ ions in the immediate vicinity of roots is reduced and diffusion gradients are established.
The effect of submergence on the chemistry and availability of soil potassium has not been studied sufficiently.
Potassium is present in soils in four forms, which are in dynamic equilibrium as follows:
With flooding or submergence soluble ferrous (Fe2+) and manganous (Mn2+) ions increase and exchangeable K+ is then displaced into the soil solution. The increase in soluble K+ after submergence is closely related to the ferrous ion (Fe2+) content of the soil solution.
The release of K+ from micas may be the contributing factor for the increase in K+ in soil and that release depends on the various factors like tetrahedral rotation, degree of tetrahedral tilting, —OH groups orientation, degree of K+ depletion from the soil solution, hydronium ions (H3O+), biological activity and complexing organic acids, inorganic cations etc. Besides these, charge density and the configuration of the oxygen about exchange sites probably determines the release of K+ and thus increases the concentration of K+ in the soil solution.
It is evident that rice plants can absorb a larger percentage of the total absorbed K+ from the non-exchangeable form under submergence than that of non-submergence conditions. Continuous submergence and alternate drying and wetting increased the exchangeable potassium (K+) content.
It has been also reported that the availability of applied potassium decreases in submerged soils due to formation of Fe-K sparingly soluble complexes.
Nutrient Element # 4. Sulphur:
In submerged soils the main transformations of sulphur are the reduction of sulphate (SO42-) to sulphate (S2-) and the dissimilation of the amino acids, cysteine, cystine and methionine to H2S. Methyl-thiol has been found in submerged soils and the bad odour of putrefying blue-green bacteria in a reservoir has been attributed to dimethyl sulphide and methyl, butyl and isobutyl thiols.
The main product of transformations of the sulphur in submerged soils is H2S and it is derived largely from SO42- reduction. After formation of H2S due to SO42- reduction results from the soil submergence, it may react with various heavy metals (Zn, Cu, Cd, Pb etc.) to give their insoluble sulphides.
As a result the availability of these metals may be reduced. Because Fe3+ reduction to Fe2+ precedes SO42- reduction, there usually will be Fe2+ present in the soil solution by the time hydrogen sulphide (H2S) is produced and to that hydrogen sulphide will be converted to insoluble iron sulphide (FeS).
In submerged soil
This above reaction protects micro-organisms and higher plants from toxic effects of hydrogen sulphide (H2S). In muck and sandy soils low in iron, however, where iron is inactivated by the complex formation with organic matter, FeS formation may not take place. In that situation H2S toxicity to the rice plant is possible.
When an acid soil is submerged the concentration of water soluble SO42- increases initially and thereafter the concentration of the same decreases slowly. The initial increase in SO42- concentration is due to the release (following increase in pH) of SO42-, which is strongly sorbed at low pH by clay and hydrous oxides of Fe and Al.
Rice, like other plants, absorbs sulphur primarily in the forms of SO42- and the reduction of SO42- to sulphide (S2-) in submerged soils reduces the availability of sulphur. However, rice takes up sulphur which is oxidised as SO42- on the root surface.
The reduction of SO42- has three implications:
(i) Sulphur supply may become insufficient,
(ii) Zinc and copper may be immobilized and
(iii) H2S toxicity may arise in soils low in iron.
Nutrient Element # 5. Iron:
The most important chemical change that takes place when a soil is submerged is the reduction of iron and the accompanying increase in its solubility. The intensity of reduction depends upon time of submergence, amount of organic matter, active iron, active manganese, nitrate etc.
Due to reduction of Fe3+ to Fe2+ on submergence, the colour of soil changes from brown to grey and large amounts of Fe2- enter into the soil solution. It is evident that the concentration of ferrous iron (Fe2+) increases initially to some peak value the thereafter decreases slowly with the period of soil submergence. Organic matter also enhances the rate of reduction of iron in submerged soils.
The initial increase in the concentration of ferrous iron (Fe2+) on soil submergence is caused by the reductions that are shown below:
The decrease in the concentration of Fe2+ following the peak rise is caused by the precipitation of Fe2+ as FeC03 in the early stages where high partial pressure of CO2 prevails and as Fe3(OH)8 due to decrease in the partial pressure of CO2(pCO2)
Rice benefits from the increase in availability of iron but may suffer in acid soils, from an excess.
The reduction of iron has some important consequences:
(i) The concentration of water soluble iron increases,
(ii) pH increases,
(iii) Cations are displaced from exchange sites,
(iv) The solubility of P and Si increases and
(v) New minerals are formed.
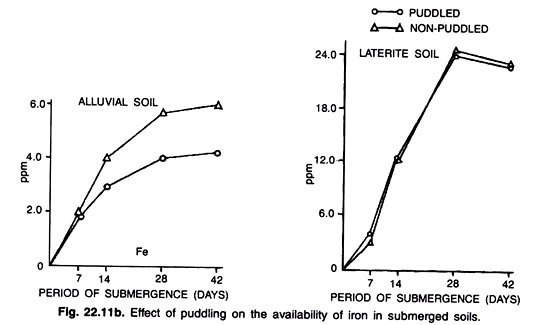
For an example, experimental evidence on the transformation of DTPA-extractable (Diethylenetriaminepenta acetic acid) Fe under different moisture regimes and times of organic matter (well rotten FYM) application in both puddled and non-puddled alluvial soils of West Bengal is depicted in figures (22.11a and 22.1 lb).
From the above figure it is observed that the magnitude on release of DTPA-extracted Fe from the soil was found to be higher under waterlogged and puddled soil conditions. On the other hand, the amount of extractable Fe was found to be maintained a higher value in which organic matter (@ 1 per cent by weight of soil well rotten FYM) was applied 14 days before puddling the soil throughout the period of submergence.
A schematic representation for the transformation of iron in submerged soils is shown below:
Nutrient Element # 6. Manganese:
The main transformations of manganese in submerged soils are the reduction of manganic (Mn4+) to manganous (Mn2+) and almost similar to that of iron transformation. Like iron, the transformation for Mn is also governed by the redox equilibria system.
In submerged soils, the transformation of Mn results an increase in the concentration of water soluble Mn2+, precipitation of manganous carbonate (MnCO3), and re-oxidation of Mn2+ diffusing or moving by mass flow to oxygenated interfaces in the soil.
When an aerobic laterite soil is submerged the reduction of manganic manganese (Mn4+) occurs almost concurrently with the nitrate (NO3–) reduction, but this reduction precedes that of Fe reduction. The concentration of Mn2+ (water soluble) increases initially and thereafter declines with the period of soil submergence that is shown in (Fig. 22.13).
The initial increase in the concentration of Mn2+ may be due to the reduction of soil as well as Mn4+ and the decrease of the same of the later period may be due to the precipitation of Mn2+ on MnCO3 and Mn(OH)2 in the soil solution.
The kinetics of manganese reduction varies markedly from soil to soil. The changes in water soluble Mn2+ concentration depend upon the pH, organic matter content and active Mn content of the soils. The mobilization of Mn in soils is markedly increased after submergence due to the reduction of manganic compounds to more soluble forms as a consequence of the anaerobic metabolism of soil bacteria.
Acid lateritic soils high in active Mn regardless of organic matter content will give higher peak of water soluble Mn2+ concentration sharply and low organic matter content delayed the peak. Strongly acid soils with relatively low in Mn content will also give lower peaks. The smallest peak will produce in slightly alkali soils and in soils very low in Mn content.
We know that the transformation of Mn in submerged soils largely depends on the oxidation-reduction reactions and the reduction of Mn4+ occurs when the redox potential value is within a range from +200 to +400 mV.
It is evident that organic matter influences the manganese transformation in soils through the following ways:
(i) The production of complexing agents that effectively reduces the activity of free iron in solution.
(ii) The decrease in the oxidation-reduction potential of the soil either directly or indirectly through microbial activity.
(iii) The stimulation of microbial activity that results in the incorporation of Mn in biological tissue.
Nutrient Element # 7. Zinc:
The transformation of zinc in submerged soils is not involved in the oxidation-reduction process like that of iron and manganese. However, the reduction of hydrous oxides of iron and manganese, changes in soil pH, partial pressure of CO2, formation insoluble sulphide compound etc. In soil on submergence is likely to influence the solubility of Zn in soil either favorably or adversely and consequently the Zn nutrition of low and rice.
The reduction of hydrous oxides of iron and manganese, formation of organic complexing agents, and the decrease in pH of alkaline and calcareous soils on submergence are found to favour the solubility of Zn, whereas the formation of hydroxides, carbonates, sulphides may lower the solubility of Zn in submerged soils. Zinc deficiency in submerged rice soils is very common owing to the combined effect of increased pH, HCO3– and S2– formation.
The solubility of native forms of Zn in soils is highly pH dependent and decreases by a factor of 102 for each unit increase in soil pH. The activity of Zn-pH relationship has been defined as follow:
Soil + Zn2+DSoil – Zn + 2H+
The pK value for the above reaction with the solid phase of soils is 6.0. This equation holds good for submerged soils.
Some equations relating to solubility of Zn in submerged soils governed by various metastable compounds are given below:
Many of these compounds are metastable intermediate reaction products and varying mean residence time in submerged soils. Applied Zn tends to approach the solubility of the native forms instead of having residual effect in the former Zn forms.
When an aerobic soil is submerged, the availability of native as well as applied Zn decreases and the magnitude of such decrease vary with the soil properties. The transformation of Zn in soils was found to be greatly influenced by the depth of submerged and application of organic matter.
If an acid soil is submerged, the pH of the soil will increase and thereby the availability of Zn will decrease. On the other hand, if an alkali soil is submerged, the pH of the soil will decrease and as a result the solubility of Zn will generally increase.
The availability of Zn decreases due to submergence may be attributed to the following reasons:
(i) Formation of insoluble franklinite (ZnFe2O4) compound in submerged soils.
(ii) Formation of very insoluble compounds of Zn as ZnS under intense reducing conditions,
(iii) Formation of insoluble compounds of Zn as ZnCO3 at the later period of soil submergence owing to high partial pressure of CO2(pCO2) arising from the decomposition of organic matter,
(iv) Formation of Zn(OH)2 at a relatively higher pH which decreases the availability of
(v) Adsorption of soluble Zn2+ by oxide minerals e.g. sesquioxides, carbonates, soil organic matter and clay minerals etc. decreases the availability of Zn, the possible mechanism of Zn adsorption by oxide minerals is shown below:
Mechanism I:
In mechanism I, Zn2+ adsorption occurs as bridging between two neutral sites, but in addition to this mechanism, Zn2+ could also be adsorbed to two positive sites or to a positive and neutral site.
Mechanism II:
This mechanism occurs at low pH and results non-specific adsorption of Zn2+. In this way Zn2+ is retained and rendered unavailable to plants.
(vi) Formation of various other insoluble zinc compounds which decreases the availability of Zn in submerged soil e.g. high phosphatic fertilizer induces the decreased availability of Zn2+,
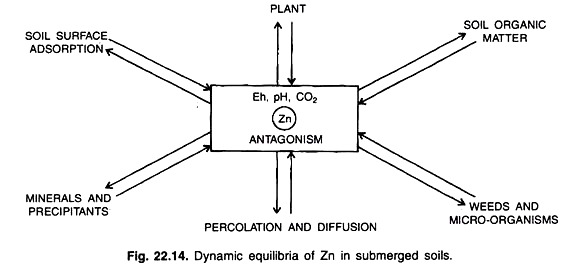
A simplified diagram illustrating dynamic equilibria of Zn in submerged soils is shown in Fig. 22.14.
It shows that rice receives Zn from the soil solution and the exchangeable and adsorbed solid phase including the soil organic fractions.
Zinc sulphide (ZnS, Sphalerite) in the presence of traces of hydrogen sulphide (H2S) in submerged soils may control the solubility of Zn. Zinc is stable in submerged soils. So it can be concluded that higher the pH and poorer the aeration, the greater is the likelihood of Zn deficiency if the soil solution Zn activity is controlled by sphalerite (ZnS).
Therefore, a variety of chemical reactions in soils influence the availability of Zn to rice. For example, high manganese concentration antagonises Zn absorption and translocation. Calcium and magnesium may also affect Zn uptake.
The reversible pH change of the submerged soils, where the pH tends to increase in acid soil and decrease in alkaline soils, undoubtedly modify the Zn equilibrium concentration in the soil solution. Because the solubility of Zn minerals and Zn sobered by soil colloids is pH dependent (higher at higher pH), an increase in the pH of an acid soil when submerged will tend to decrease the Zn concentration in the soil solution.
In alkaline soil, however initially Zn uptake increases as the pH decreases after submergence. Submerged alkali or calcareous soils possess all the essential characteristics for the formation of high amount of bicarbonate (HCO3-) ions which helps Zn2+ rendering unavailable to plants by forming insoluble ZnCO3 compound. Experimental evidence indicating the transformations of Zn2+ under different soil and water management practices in laterite and alluvial soils of West Bengal is illustrated in figures 22.15a and 22.15b.
In addition to these, the availability of Zn in submerged soils is governed by the mutual interaction of quantity (q) intensity (c), and kinetic parameters as regulated by the adsorption, desorption, chelation and diffusion of Zn from soils to the plant roots. The quantity-intensity relationship of Zn in submerged soils may be described by the linear form of the Langmuir type equation. The supply parameter assumes the form,
where q is the quantity c is the intensity, K1 and K2 are constants.
The optimum Zn supply to rice is ensured when the value of the supply paratmeter is unity (1.0).
Different crop management factors combinedly influence the availability of Zn to rice like, native Zn content of the soil, soil pH, organic matter, submergence, partial pressure of CO2, HCO3–, organic acids, various natural interactions, environmental effects and water quality, etc.
Nutrient Element # 8. Copper:
Most of the copper in soils is very insoluble and can only be extracted by strong chemical treatments which dissolve various mineral structures of solubilize organic matter. The concentration of copper in soil solutions is usually very low. Al pH values below 6.9, divalent Cu2+ is the dominant species. Above pH 6.9, Cu(OH)20 is the principal solution species and CuOH+ at pH 7.0.
Hydrolysis reactions of copper ions are shown below:
The complexes CuSO40 and CuCO30 are also important forms of copper. Solubility of copper is very pH dependent and it increases several times (approx. 100 times) for each unit decrease in soil pH. The transformation of copper in submerged soils is not involved in oxidation-reduction reactions; its behaviour is influenced by simple submergence in soils.
It is evident that copper exists in soils as different discrete chemical pools which are as follows:
(i) Water soluble plus exchangeable Cu.
(ii) Copper associated with clay minerals.
(iii) Organically bound Cu.
(iv) Copper associated with different oxides in soils.
(v) Residual copper.
The amount of each form of copper in soils depends on soil pH, amount of organic matter, clay content, oxides of Fe and Mn etc. All these above forms of Cu are in dynamic equilibrium in soils.
In submerged soils, copper comes into the soil solution or available pool and becomes available to the plant as follows:
The chemical equilibria of Cu in submerged rice soils are similar to those of Zn. The mechanism for removal of Cu from soil solutions in submerged soils is so pronounced that copper is apparently removed from chelating agents that is capable of keeping the element is solution phase in upland soils.
When an acid soil is submerged, the release of copper decreases due to increase in soil pH, whereas submerging an alkali and calcareous soils, the amount of copper in soil solution increases to a lesser degree. However, in most of the soils, submergence decreases the availability of copper and thereby creates deficiency to plants.
The possible explanations for the increase in the concentration of copper in submerged soils are formation of organic complexes and decreased soil pH (alkali soil). On the other hand, the decrease in the amount of copper may be due to the insoluble precipitation as CuS, CUCO3 and Cu(OH)2 since the production of sulphide, carbonate, bicarbonate and hydroxide is more in submerged soils resulting from the reduction of soils.
Again submerging a soil high in organic matter, the amount of extractable Cu either decreases or increases and it is contradictory. The decrease may be due to the microbiological immobilization and the antagonistic effect of increased concentration of iron, manganese and phosphorus forming insoluble copper complexes in soils.
It is also evident that the higher concentration of phosphorus in submerged soil decreased the availability of copper. The possible mechanism for enhanced copper retention on allophane and oxides, in which phosphate coordinates to the axial position of a surface bound copper (Cu2+) ion, thereby produces a ternary surface copper complex.
Evidently due to application of organic matter in submerged soils the amount of available copper increases. The increase may be due to the reduction of coating of hydrous oxides of Fe3+ and Mn4+ on the copper compounds and also for the production of soluble Cu-organic chelates and thus increases its solubility.
An experimental evidence for the changes in the availability of copper under soil and water management practices in soils of West Bengal is illustrated diagrammatically in Figures 22.16a and 22.16b.
Nutrient Element # 9. Boron and Molybdenum:
Very little work has been done so far on the chemical equilibrium of boron and molybdenum in submerged soils. Since the solubility of the oxyanionic forms of these two elements is very much dependent on pH, organic matter content, clay content etc. Their availability in submerged soils is related to the changes in soil pH.
Submerging an acid soil caused an increase in the amount of available Mo content during the initial period, which remained almost unchanged at the later period. This increase might be due to the increase in soil pH and desorption of MoO42- from oxides and hydroxides of Fe and Mn. It has been reported that the concentration of B in soil solution remains more or less constant after submergence.